Blog Posts
Explore timely perspectives from WCG’s clinical trial operations and scientific leaders.

WCG Clinical | Insights
What ethical safeguards are needed for research involving observation of group behavior?
Blog Posts
WCG Clinical | Insights
Questions on the FDA’s 30-Day IND Review Period and IRB Approval
Blog Posts
WCG Clinical | Insights
NIH Launches NExTRAC to Advise on Emerging Biotechnologies
Blog Posts
WCG Clinical | Insights
WCG’s Clinical Research Trends & Insights for 2020
Blog Posts
WCG Clinical | Insights
How to Maximize ROI by Implementing a Holistic Recruitment Strategy
Blog Posts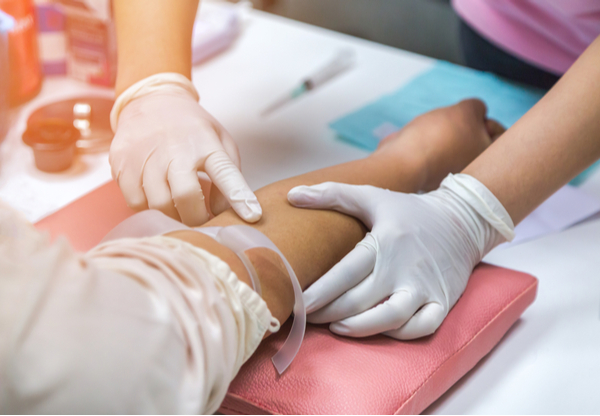
WCG Clinical | Insights
What are the Blood Draw Guidelines for Phase 1 Clinical Trials?
Blog Posts
WCG Clinical | Insights
Questions on Using Generic Recruitment Flyers at Clinical Trial Sites
Blog Posts
WCG Clinical | Insights
An Overview of the Recent SACHRP Recommendations Around Payments in Clinical Research
Blog Posts
WCG Clinical | Insights
Creating Scientific Rigor in Medical Device Trials
Blog Posts
WCG Clinical | Insights