Driving Research to Results:
The trusted partner for 94% of all FDA-approved therapeutic agents in the last five years.
Transforming Clinical Research for Success
Knowing Changes Everything
WCG’s Data and Insights solutions bring regulatory, operational, and performance intelligence together to give sponsors a unified, evidence-based view of trial performance. Learn how Knowing Changes Everything when you work with WCG.
Design Smarter Research with Expertise Where it Matters
Our connectivity, solutions, data intelligence, and expertise support your success.
integrated ecosystem of tech-enabled solutions and intelligence driven by data, powered by AI.
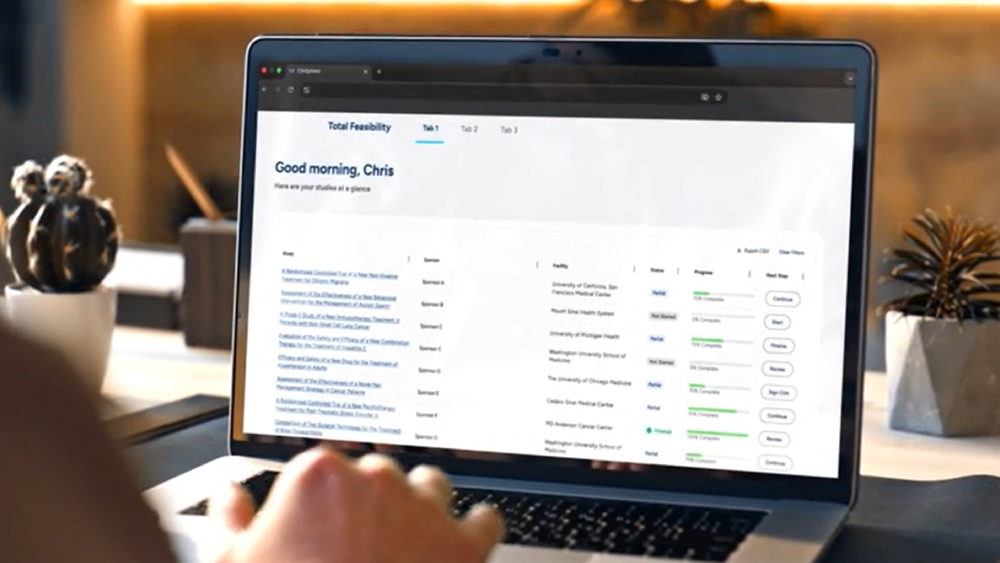
Explore WCG ClinSphere™
protocol dataset—the industry's largest— combined with unmatched performance and operational data to enable smarter research.
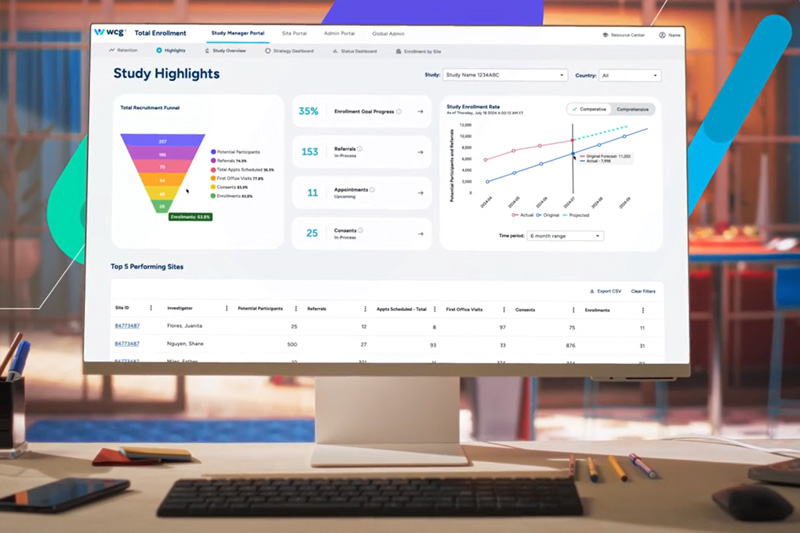
Explore Data & Insights
multi-stakeholder consortium, co-led with the MRCT Center, to address ethical challenges in AI-based clinical research.
What’s Slowing Down Your Clinical Trials?
Long study start-up and trial duration?
- Our average full-board IRB review determinations arrive 20% ahead of targeted TAT, accelerating site activation and study start-up.
- Complete study start-up 37% faster, and connect with the right sites with our leading database of historical performance for sites and investigators.
Explore our solutions for:
Increased competition for sites and participants?
- 80% of all clinical trials fail to meet patient enrollment targets. Improve your recruitment and retention with our solutions.
- Share knowledge with 220+ peer organizations in a collaborative, pre-competitive consortium, while driving efficiencies with 500+ best practice tools.
Explore our solutions for:
Increased site burden and protocol complexity?
- Clinical research sites can reduce overall time to study activation by up to 50% by leveraging WCG’s Site Enablement Solutions.
- Clinical research sites can decrease their budget and contract negotiation timelines by up to 40-60% following WCG’s industry-leading best practices.
Explore our approach to:
In Focus Topics
Clinical Trial Operations
Artificial Intelligence in Clinical Trials
Regulatory Compliance
Clinical Trial Safety
Participant Recruitment
Founder of independent ethical review and trusted partner for 55+ years
The clinical trial industry faces strong headwinds – new trial designs, too many technology platforms, greater complexity – all of which requires a new, more modern approach. WCGs global reach and purpose-built solutions are designed to connect sponsors, CROs, sites, and participants across the industry to navigate the future of clinical research and help bring life-saving therapies to patients faster.
Upcoming
Events
Join our thought leaders for deeper dives into the clinical trial industry.
Driving Research to Results
Mid-sized BioPharma Closes Enrollment 3 Months Ahead of Schedule
- Enrollment closed nearly 3 months ahead of schedule, which had only seemed impossible.
- The client was able to bolster enrollment because the media outreach came in under budget and funds were reallocated to site support.
- Additionally, 118 of the 272 documents reviewed were given suggestions for improvement in accordance with ICH (E6) R2 and industry-leading practices.
WCG has been an important partner to our company in providing strategic expertise and development support in challenging therapeutic areas. Their consultative services, particularly around accurate collection of patient reported outcomes and placebo response mitigation, are incredibly valuable to our programs.”
Senior Medical Director, Top Global Biotechnology Company
Your knowledge, experience and professionalism contributed greatly to the success of our recent proof-of-concept trial. Working together, we produced a high-quality trial with robust findings that helped guide program decision-making. I greatly enjoyed and appreciated partnering with you on this project.”
Clinical Team Lead, Top 10 Sponsor Company
A few of the places our scientific and clinical operations thought leaders are featured: