Research Sponsors
WCG Clinical | Insights
What are the requirements for submitting protocol deviations?
Blog Posts
WCG Clinical | Insights
Podcast: The Role of Expert Committees in Clinical Research with guest Jonathan Seltzer, MD, MBA, MA, FACC
Podcasts
WCG Clinical | Insights
Eliminating the Headache of Global Safety Reporting to Investigators and Ethics Committees
Videos
WCG Clinical | Insights
What elements of Informed Consent must we include when pre-screening?
Blog Posts
WCG Clinical | Insights
The Role of Expert Committees: An Interview with Dr. Seltzer
Videos
WCG Clinical | Insights
Does the IRB need to review news stories?
Blog Posts
WCG Clinical | Insights
Protecting Sponsors Against Bias and Variability
Articles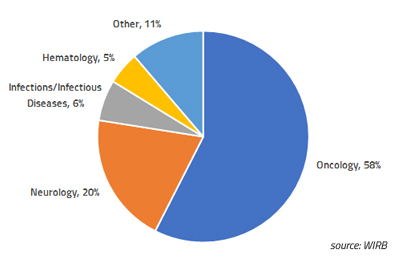
WCG Clinical | Insights
Single-Patient Expanded Access: WIRB experience in 2018
Blog Posts
WCG Clinical | Insights
How a Sponsor Secured 200 High-Performing PI’s in a New TA in Just 5 Days
Case Studies
WCG Clinical | Insights