Regulatory Compliance

WCG Clinical | Insights
What Information Must Be Included in the Cost Section of an Informed Consent Form?
Blog Posts
WCG Clinical | Insights
Why Was My Research Submission/Protocol Deferred? And What Should I Do?
Blog Posts
WCG Clinical | Insights
How New Changes to the NIH Guidelines Will Impact IBC Review
Blog Posts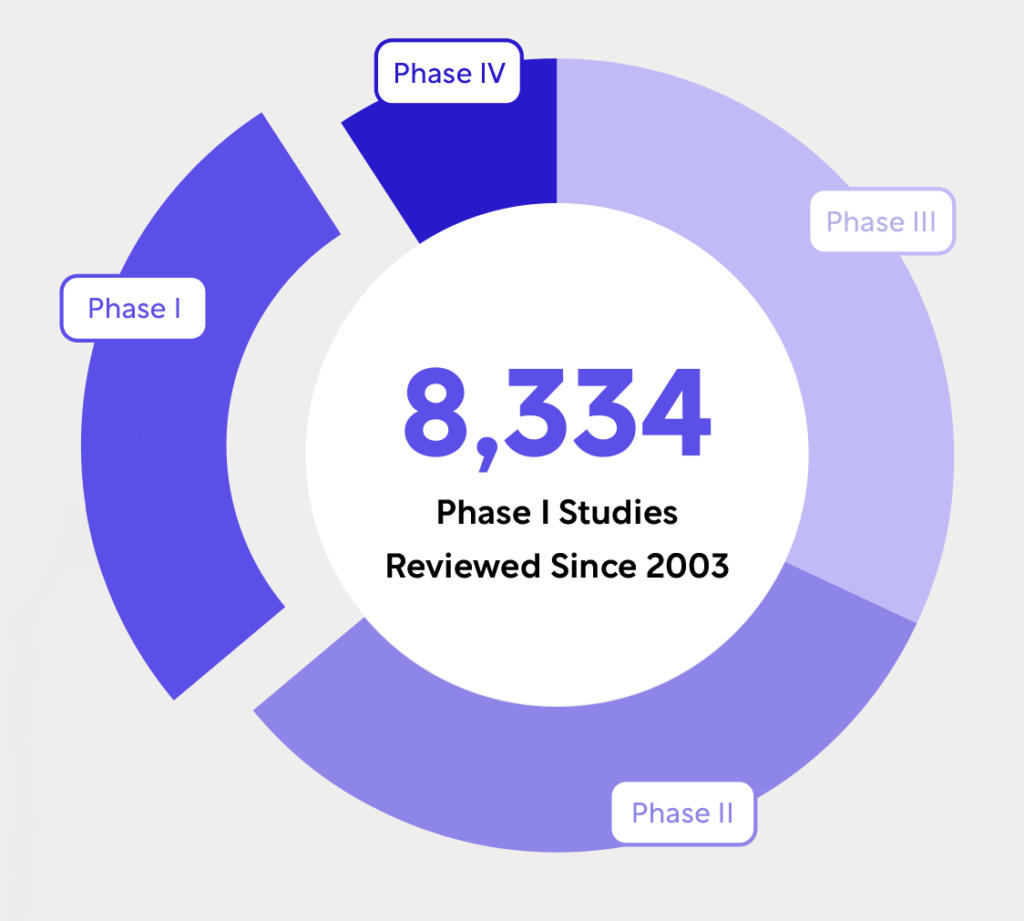
WCG Clinical | Insights
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
WCG Clinical | Insights
What You Should Know About FDA Final Informed Consent Guidance
Blog Posts
WCG Clinical | Insights
The Nuances of Patient Selection: Why Some Trials Need Eligibility Adjudication
Whitepapers
WCG Clinical | Insights
Function over Form: Assessing Different Consent Form Formats
Whitepapers
WCG Clinical | Insights
Why Biotech Sponsors Need Outside Support: IRB, IBC, DMCs and EACs
Whitepapers
WCG Clinical | Insights
Which comes first – IRB or IBC approval?
Blog Posts
WCG Clinical | Insights