Are you looking at trial budgeting through a real-world lens? Ideally, you receive the sponsor’s budget table, and your site has a standard cost for every protocol item. You send your site’s cost request to the sponsor to initiate negotiations, and the sponsor accepts all budget requests within 30 days.
In reality, most sites see at least one round of negotiation involving phone calls, clarification emails, and justification requests. Prolonged negotiations can lead to study delays. How can you streamline the budgeting process? Here are our top five tips:
1. Standard Fee Schedules – Have a standard fee schedule in place to ensure budget quality and have cost documentation available before negotiations begin. Standard items include:
- Time and effort
- Hourly rates for study staff budgeting
- Baseline time estimates for common activities
- Patient care costs
- Chargemaster/s – single, research-specific or multiple
- Clear Medicare pricing documentation
- Contractor rates for routine outside services
- Bottom-line and negotiation thresholds for items in the fee schedule
- Administrative fees
- Standard fee schedule per department
- Study-level internal site fees
- Non-negotiable fees
2. Study-Specific Considerations – Before building the budget, have the specific start-up processes documented for each study, including:
- Coverage analysis, if required
- Details from coverage analyst
- Notes from the study team with special requirements
- Departmental protocol reviews
- Mirror recently negotiated budget
- Confirmation of budget contacts
3. Internal Budget Development – Each site’s internal budget development process may look slightly different so don’t rely on a general template, but these tactics can pay off:

Work with the study team to identify activities with special timing or staffing requirements. They should confirm requirements for activity, including heavy visits, such as extended PK sampling days, observation days, or protocols requiring inpatient stays. Also include data management costs and ensure that you have appropriate PI and coordinator time allocated.
4. Budget Negotiation Expectations – Work with the sponsor/CRO to set clear expectations for the budget negotiation process with these steps:
- Include an ideal timeline for completion.
- Create site standards for follow-up timelines.
- Be prepared for a counteroffer below your bottom line.
Prepare FAQs for overhead, fair market value, IRB fees, and start-up costs. Having documentation of start-up costs will help fast-track the process. Negotiate for start-up payments to be made automatically upon execution to save administrative time and effort.
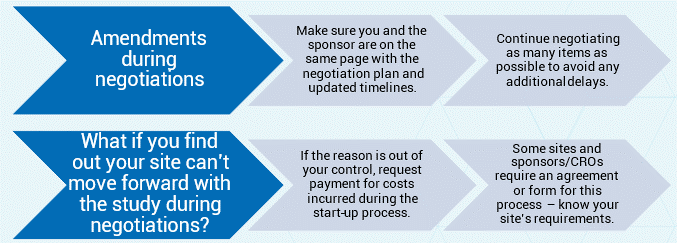
5. Amendments & Cancellations – Understand your site’s process and standards for when amendments and/or cancellations happen:
Conclusion
Using these five budget development tips can help sites prepare for budget challenges, avoid delays, and encourage smoother negotiations. By reaching an ideal but realistic budget, sites can help expedite the study start-up process and get patients the treatment they need in a more timely manner.
Want to learn more about how to improve budget negotiations at your research site? Connect with our experts today to get started.
Do you want to improve your site's financials and find revenue you are owed?
Complete the form to schedule a consultation with WCG.
