Blog Posts
Explore timely perspectives from WCG’s clinical trial operations and scientific leaders.

WCG Clinical | Insights
Amendments to the NIH Guidelines: Effects on Human Gene Transfer Research
Blog Posts
WCG Clinical | Insights
What elements of Informed Consent must we include when pre-screening?
Blog Posts
WCG Clinical | Insights
Does the IRB need to review news stories?
Blog Posts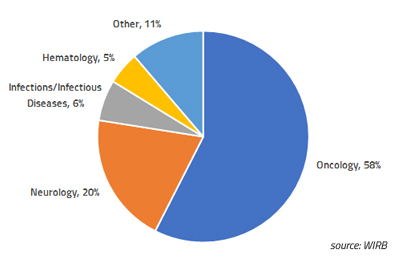
WCG Clinical | Insights
Single-Patient Expanded Access: WIRB experience in 2018
Blog Posts
WCG Clinical | Insights
Limited IRB Review: Are You Prepared for January 21st?
Blog Posts
WCG Clinical | Insights
Germline Modification for HIV Prevention is Unjustified
Blog Posts
WCG Clinical | Insights
Changes to Human Gene Transfer Oversight at NIH
Blog Posts
WCG Clinical | Insights
Gene Therapy: New Draft Guidance Will Impact Clinical Trial Designs
Blog Posts
WCG Clinical | Insights
The Eligibility Conundrum in Clinical Trial Protocols: FDA Issues Workshop Report
Blog Posts
WCG Clinical | Insights