Clinical Operations

Regulatory Compliance
What training documentation is required for conducting clinical research studies?
Blog Posts
What are ethical considerations for a trial with a pre-study washout period?
Blog Posts
Virtual Clinical Trials: Best Practices in Moving Toward a Patient-Centric Research Model
Whitepapers
Regulatory Compliance
How to resolve irregularities in the documentation of informed consent?
Blog Posts
Ethics in Clinical Research
Can the IRB approve reimbursement of copays for routine costs in a clinical trial?
Blog Posts
Regulatory Compliance
Is IRB review required for survey results that may be published?
Blog Posts
Clinical Endpoints
Eliminating the Headache of Global Safety Reporting to Investigators and Ethics Committees
Videos
Ethics in Clinical Research
Does the IRB need to review news stories?
Blog Posts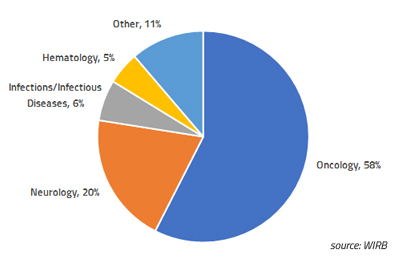
Single-Patient Expanded Access: WIRB experience in 2018
Blog Posts