Med/Sci

Use of Endpoint Adjudication to improve the quality and validity of endpoint assessment for Medical Device Development
Articles
Clinical Endpoints
Responsibilities of Data Monitoring Committees: Consensus Recommendations
Articles
Clinical Trial Safety
An Estimate of the Benefit-Cost Impact of the FDA Guidance on Data Monitoring Committees
Articles
Site Efficiency
Operationalizing the NIH Single IRB Mandate
Videos
Ethics in Clinical Research
Applied Clinical Trials: Single IRB Review for All Multicenter Clinical Trials
Articles
Ethics in Clinical Research
IRB Advisor: Newest Oncology Studies Raise Ethical, Other Questions for IRBs
Articles
Clinical Researcher: Applying ISO9001 to the IRB Process
Articles
Subject Recruitment Materials: Understanding the Requirements for IRB Review
Whitepapers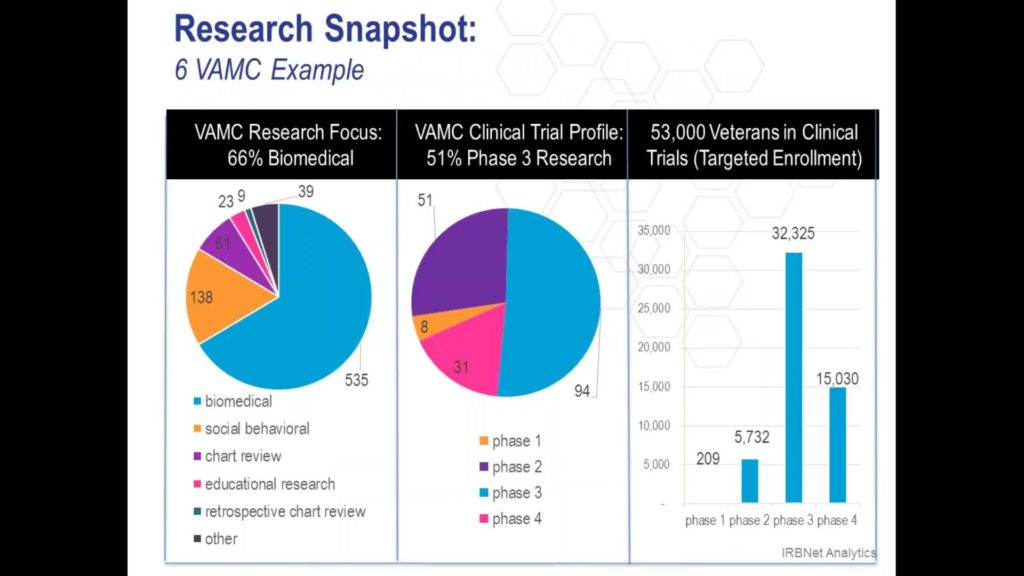
IRBNet and VA Medical Centers: A Powerful Partnership Made Even Easier
Videos