Blog Posts
Explore timely perspectives from WCG’s clinical trial operations and scientific leaders.
What are the requirements for submitting protocol deviations?
Blog Posts
Regulatory Compliance
Can we transfer patient data from our CTMS to our parent medical practice?
Blog Posts
Amendments to the NIH Guidelines: Effects on Human Gene Transfer Research
Blog Posts
What elements of Informed Consent must we include when pre-screening?
Blog Posts
Ethics in Clinical Research
Does the IRB need to review news stories?
Blog Posts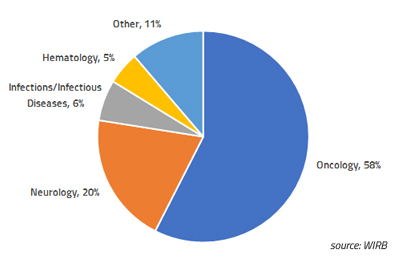
Single-Patient Expanded Access: WIRB experience in 2018
Blog Posts
Limited IRB Review: Are You Prepared for January 21st?
Blog Posts
Germline Modification for HIV Prevention is Unjustified
Blog Posts
Changes to Human Gene Transfer Oversight at NIH
Blog Posts