Insights
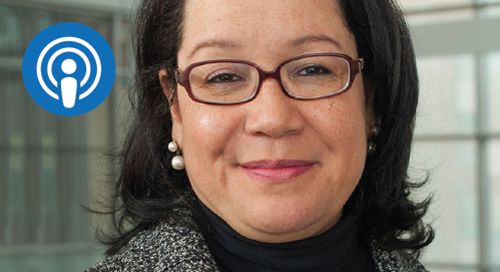
COVID-19
Jonca Bull, MD: We Should Not Be Surprised — COVID-19 Disparities Lay Bare What’s Been There All Along
Podcasts
COVID-19
PART 5 – Clinical Trials in the Era of COVID-19: Changes You Need to Make Now
Videos
COVID-19
Dr. Edith Mitchell, MD: COVID-19 Doesn’t Play Fair — What We Need to Understand About Racial Disparities
Podcasts
COVID-19
PART 4 – Clinical Trials in the Era of COVID-19: Changes You Need to Make Now
Videos
Metrics Champion Consortium Unveils New Forum to Foster Dialogue on Navigating Clinical Trial Operational Challenges Amid COVID-19 Crisis
News
COVID-19
Your Well-Being May Depend on a Clinical Trial: What You Need to Know
Blog Posts
COVID-19
PART 3 – Clinical Trials in the Era of COVID-19: Changes You Need to Make Now
Videos
COVID-19
Dr. David Fajgenbaum, MD, Castleman’s Disease Survivor, Facing COVID-19 with Research, Action, and Hope
Podcasts
Technology
Best Practices For Delivering Investigator Training Via Virtual Meetings
Blog Posts
COVID-19