Insights

Ethics in Clinical Research
If My Study Includes Approved Drugs, When Do the Risks of Those Drugs Need to Be Disclosed in the Consent Form?
Blog Posts
Diversity & Inclusion
Cultivating Diversity and Inclusion in Clinical Research: A Long-Term Commitment
Blog Posts
Myth vs Reality: Understanding Suicidality Risk in All Clinical Trials
Blog Posts
Diversity & Inclusion
FDA’s Path Toward Diversity in Clinical Trials: The DEPICT Act and Sponsor Responsibility
Whitepapers
Diversity & Inclusion
The Importance of Diversity, Equity, Inclusion, and Intersectionality in Clinical Research
Podcasts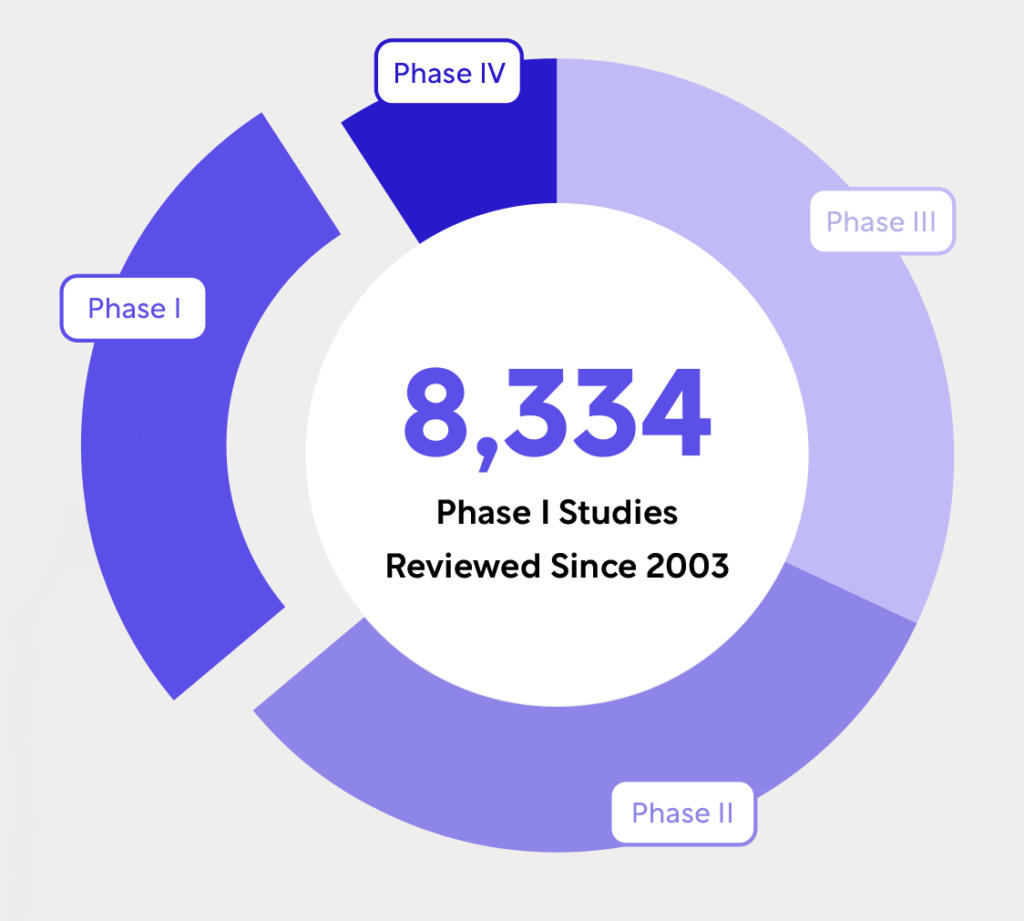
Ethics in Clinical Research
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
The Expanded Brief Assessment of Cognition (BAC) for the Assessment of Cognitive Impairment in Mild Alzheimer’s Disease
Research
Series: WCG Patient Forum
New Study for Policy Makers Documents the Burden of the Diagnostic Odyssey on Rare Disease Families
Videos
Clinical Trial Operations
Streamline Clinical Trials with InvestigatorSpace®: Harmonized Safety Report Distribution and Training
Blog Posts
Series: WCG Patient Forum