Principal Investigator

The 2025 Avoca State of the Industry Report: Understanding Adoption & Implementation of ICH E6(R3)
Reports
Site Efficiency
WCG’s 2024 Clinical Research Site Challenges Report
Whitepapers
Ask the Experts: Your Coverage Analysis & Billing Compliance Questions Answered
Videos
Ethics in Clinical Research
What Is Assent, When Provided by an Adult Participant Lacking Capacity? And What Are WCG’s Expectations and the Investigator’s Responsibilities?
Blog Posts
Series: WCG Talks Trials
Amplifying Patient Voices to Advance Clinical Research: The Importance of Patient Advocacy
Podcasts
Diversity & Inclusion
The Importance of Diversity, Equity, Inclusion, and Intersectionality in Clinical Research
Podcasts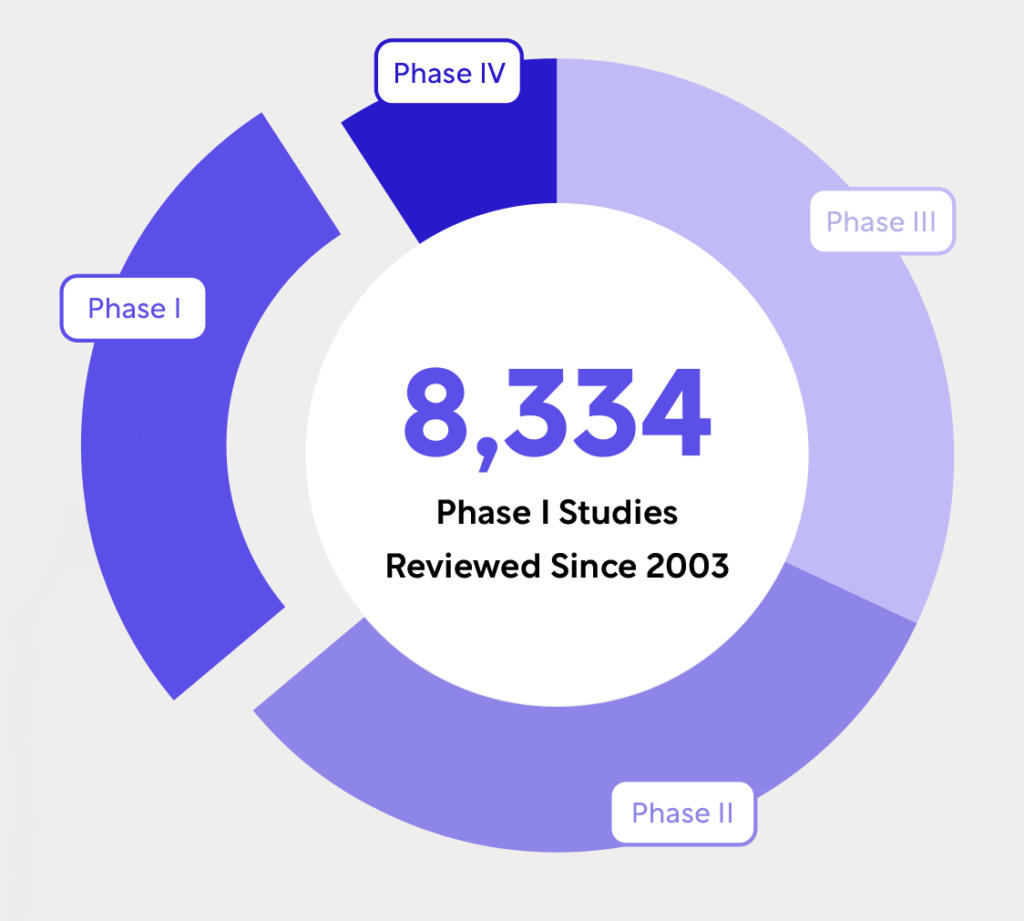
Ethics in Clinical Research
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
Clinical Trial Safety
The Nuances of Patient Selection: Why Some Trials Need Eligibility Adjudication
Whitepapers
Site Efficiency
2023 Clinical Research Site Challenges Survey Report
Whitepapers
Cell & Gene Therapy