WCG applauds the release of groundbreaking results of the National Economic Burden of Rare Disease Study. The findings were announced February 25, 2021 during a session of the Rare Disease Congressional Caucus in honor of Rare Disease Day.
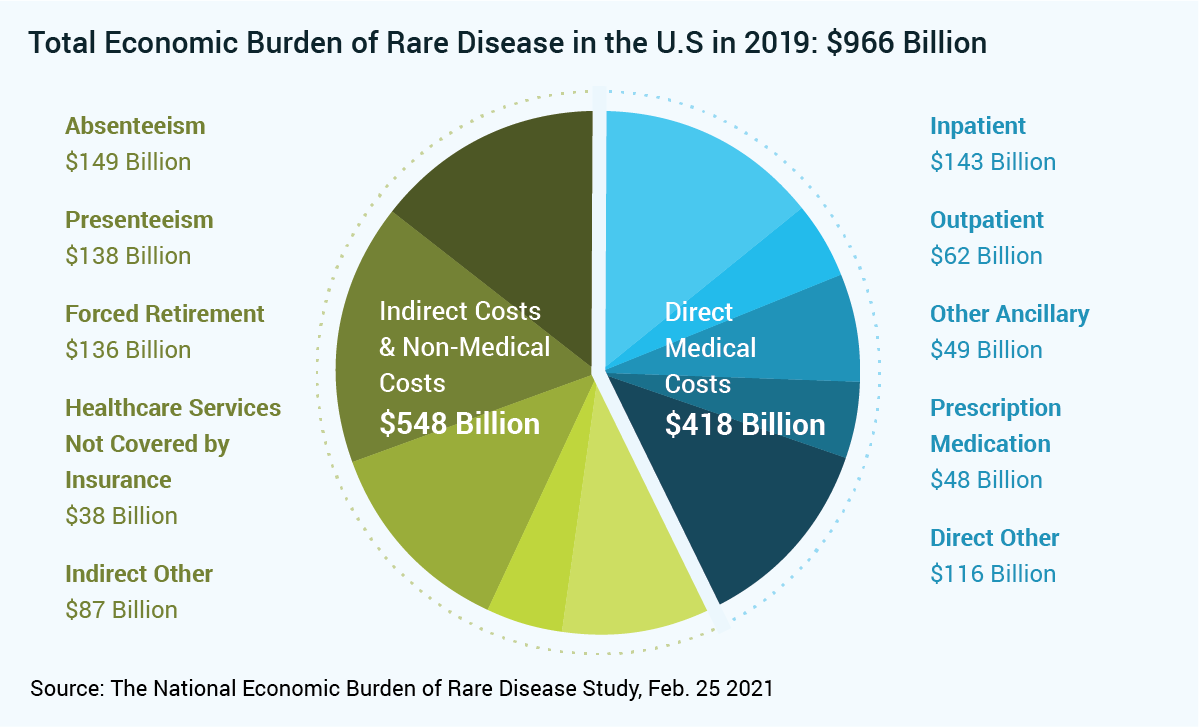
The study sheds light on the extreme economic burden rare diseases pose on families and society. In fact, the 379 rare diseases assessed in the study represent an economic burden that far surpasses even the costliest chronic diseases. Specifically, in 2019, the rare diseases evaluated affected 15.5 million individuals at a cost of $966 billion, including $418 billion in direct medical expenses and $548 billion in indirect cost.
The study was commissioned by the non-profit EveryLife Foundation for Rare Diseases, an advocate of science-driven public policy, and conducted by the Lewin Group. Armed with the knowledge that only 10% of the roughly 7000 rare diseases that affect 30 million Americans have FDA approved treatments, the National Economic Burden of Rare Disease Study was designed to:
- Provide information for policy makers, regulators, drug developers, researchers, healthcare providers, payers, and employers
- Encourage and justify policy, practice, and investment needed to reduce costly burden and pursue cures
To learn more and view the full report, please visit the study homepage at EveryLifeFoundation.org.
The chart below is adapted from Exhibit 1 in the study, modified to visually represent the leading cost categories. Note: “Presenteeism” is working while sick often causing a loss of productivity.