Where is the site voice? If you listen, you’ll hear it. A better question may be “who’s listening?”
One of the more distressing findings from the 2023 Avoca State of the Industry Report is that sites feel overlooked by sponsors.
While 65% of site staff responding to the survey agreed that sponsors design trials in a patient-centric manner, only 15% strongly agreed that sponsors take sites into consideration when designing protocols.
Among the most pressing issues, from the site perspective:
- Protocol clarity: Only 38% strongly agreed that the protocol was “clear & easy to follow.”
- Contracting: Only 31% of sites strongly agreed that budget negotiation and contracting was timely and efficient for them.
- Compensation: Only 27% strongly agreed they were “compensated fairly & on time.”
- Consideration for staff: Only 15% strongly agree that “clinical trials are designed with sites/site staff in mind.”
Among the concerns raised in the survey – in addition to lack of support from sponsors:
- Overzealous protocols
- Unclear information
- Bureaucratic start-up process
- Tight budgets
- Overly complicated and constantly amended protocols
These are not new concerns: A 2017 paper made the case that “Sites are the foundation of the clinical research enterprise, but they have surprisingly little input in the development or planning of trials.”1
It’s part of a larger disconnect. As illustrated below, sponsors and CROs fail to fully understand why sites take on studies.
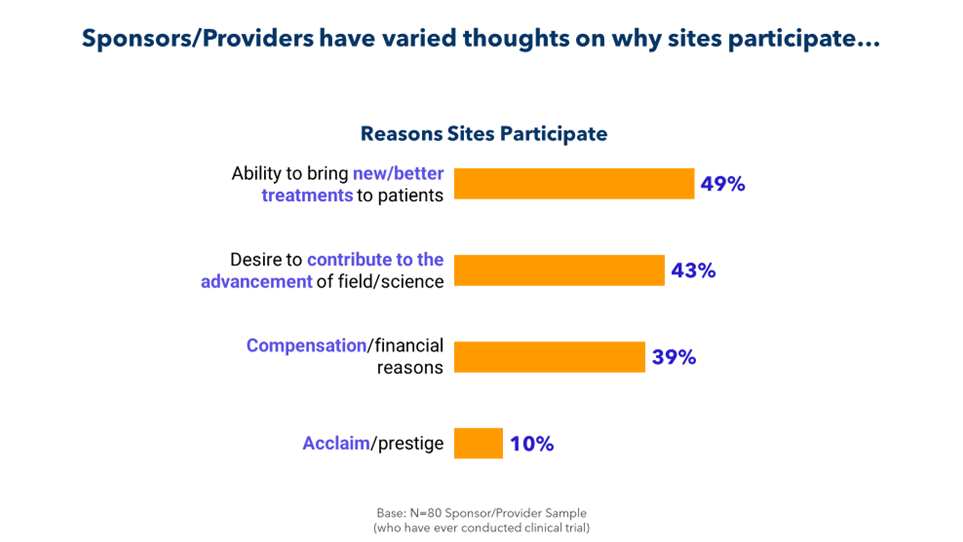
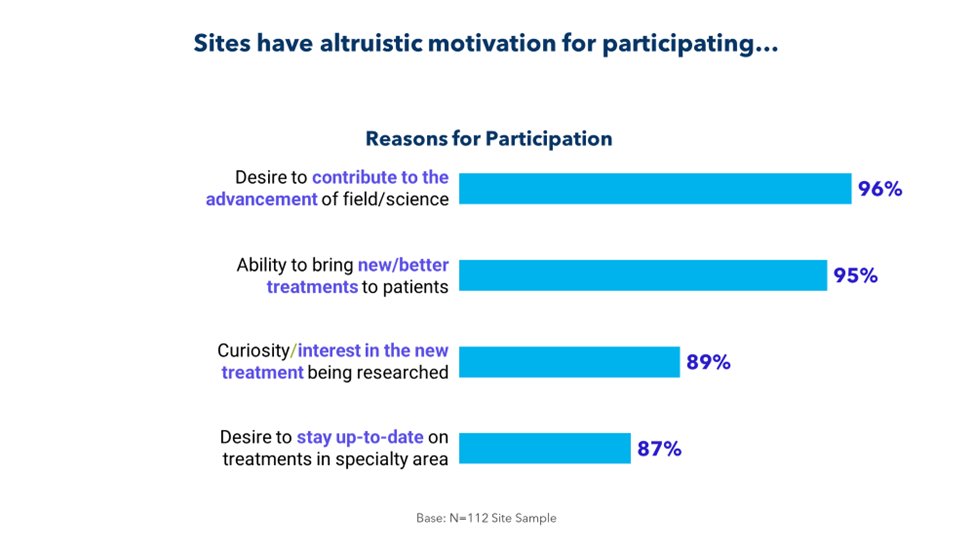
It’s time to bridge this gap for the sake of patients and for the sake of research. By collaborating more closely with sites and by soliciting and acting on feedback, sponsors and CROs can identify barriers to enrollment, retention, and recruitment; eliminate unnecessary delays; and improve the conduct of their trials.
We must capture diverse site voices in the same way we try to collect diverse patient voices. As one survey respondent put it, “Ask sites for input on your protocol. You are not an expert at boots-on-the-ground enrollment. Ask someone who is.”
One obvious but underused approach is site advisory boards.
Site Advisory Boards: Rare, but Important
Site advisory boards can be as crucial as patient advisory boards to achieving optimal, efficient trials. However, only 8% of protocols include a patient advisory board, and even fewer include a site advisory board. “Sponsors are missing an important bit of site intel,” Sandy Smith, WCG’s senior vice president of clinical solutions and strategic partnering, told CenterWatch Weekly.
Deployed correctly, these boards can effectively reduce the burden for both trial sites and patients. They can also provide sponsors and CROs with a more robust perspective on trial conduct.
“Much like we ask sponsors to get a diverse group of patient feedback when designing a protocol, we also want to get multiple diverse sites together to review protocols and the operational risk that is inherent … based on the type of site that is executing on the protocol,” Cristin MacDonald, WCG Avoca’s vice president of client delivery, told CenterWatch Weekly. “Engaging these diverse voices in the protocol design phase is imperative.”
Although a site advisory board is ideal, sponsors and CROs can find other ways to solicit feedback from those closest to the trials.
Check In, Then Listen
Perhaps the simplest thing sponsors and CROs can do is check in regularly with their sites and solicit feedback.
Starting early in the process, this engagement allows to contribute to the design and support of the protocol. These insights can help sponsors and CROs to promptly identify and address protocol design issues. As the trial progresses, this continued feedback loop enables sponsors to identify and mitigate risks in real time.
The goal is to ensure clear, timely, and effective communication with the site staff, engaging with them regularly and answering their questions promptly – and to provide training and resources as needed.
Sites can find ways to encourage sponsors to listen more closely. One option is a report card, MacDonald said. She called on sites to create a “report card” for sponsors comparing experiences across sponsors. These report cards, especially if they include data, can convince sponsors that the feedback is valid and worth acting on.
Sites and sponsors/CROs each need to make the first move to ensure site engagement. Failure to include the site voice puts patients and research at risk. It’s time for a change.
Download the full 2023 Avoca State of the Industry Report for more findings and implications for sponsors, providers, site staff, and patients.
References
- Malamis P, Howley MJ, Kremidas J. Site Perspectives on Clinical Trial Quality. Applied Clinical Trials. 2017;26(12). https://www.appliedclinicaltrialsonline.com/view/site-perspectives-clinical-trial-quality
Maximize your investment: schedule a trial design and protocol planning consultation
When you have our experts review your protocol, the early investment ensures your study—and overall investment—meets its full potential. To start, simply fill out the form.
