Despite the growing consensus around the need for diversity in clinical trials, the industry still has a long way to go.1,2 To explore this disconnect, we took a deep dive into the results of the annual WCG Avoca Industry Survey.3
One finding was particularly interesting: Respondents who saw the pursuit of diversity as a scientific or ethical imperative felt more strongly about its importance than did those who considered diversity to be important primarily for regulatory or marketing reasons.
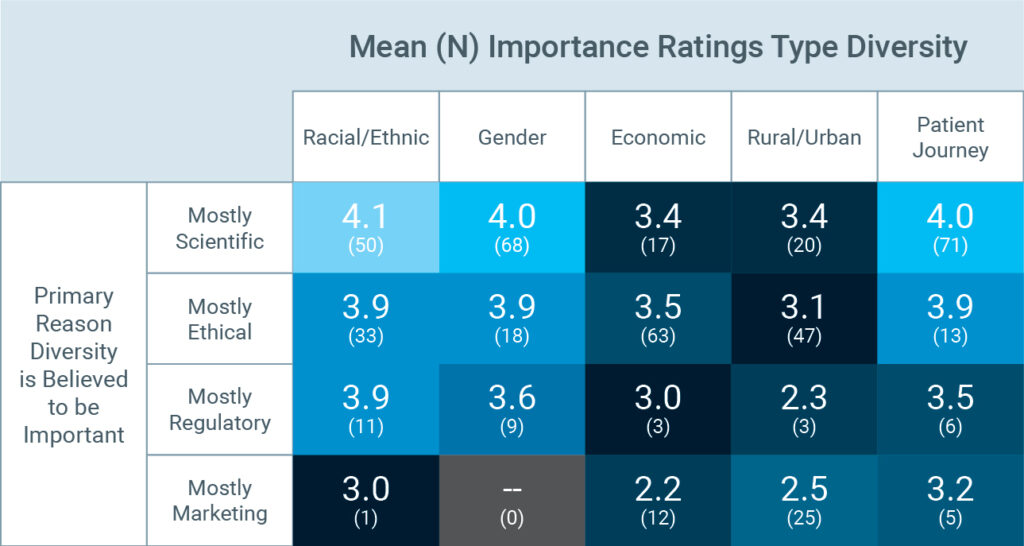
The scientific case for diversity is largely settled: What’s left is making the case. As one respondent said:
“Appropriate representation of ethnic groups and sex are critical to the scientific validity of research findings, and their absence can lead to false conclusions. Representation of patients across the spectrum of disease is crucial to ensure deep understanding of new drug therapies. While, initially, the push for diversity may be driven by the ethics of inclusion, ultimately, it benefits drug developers by…ensuring the medicines being tested work as expected in different ethnicities and at different points along the disease pathway.”
Moving decision makers away from a “check the box mentality” and ensuring they understand the scientific rationale for diversity could go a long way to improving the diversity of clinical trials in several ways:
- Generalizability: Not that long ago, researchers considered results from clinical trials to be largely generalizable to all patient populations. Growing evidence suggests this is not the case.4,5 In fact, lack of representation in clinical trials compromises generalizability of research findings.6 Trials that include diverse populations, in contrast, help ensure that the findings apply to a broader range of patients.7
- Identifying subgroup effects: Without diverse representation, potential differences in treatment response among populations may go unnoticed. Testing a drug in a diverse sample allows researchers to understand these differences, and make informed decisions about dosages, safety, and effectiveness.8
- Health equity: More robust and complete data about differences in treatment response can ultimately reduce health disparities.9
Good science is good business
Including diverse participants in clinical trials not only aligns with scientific imperatives but also has significant business benefits. Sponsors gain valuable insights into product performance among different populations, informing marketing strategies and improving market penetration.
It can also reduce development costs by minimizing the need for additional studies or post-approval research, streamlining market entry and improving profitability. However, failure to include diverse populations can result in limited evidence for effectiveness or safety, leading to label restrictions and exclusion of patient groups, ultimately hurting market share and revenue potential.
Inclusive enrollment practices can increase patient interest and public confidence in new treatments—especially when patients know the clinical trial participants look like them.10,11
Do you want help making the scientific case for DEI? Schedule a consultation with our experts today.
References:
- FDA guidance “Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry”. November 2020
- Clark LT, Watkins L, Piña IL, et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Current Problems in Cardiology. 2019;44(5):148-172. doi:https://doi.org/10.1016/j.cpcardiol.2018.11.002
- Calaprice, D, et al. Who Cares About Diversity in Clinical Trials? Journal for Clinical Studies. March 29, 2023. https://journalforclinicalstudies.com/who-cares-about-diversity-in-clinical-trials/
- Sirugo G, Williams SM, Tishkoff SA. The missing diversity in human genetic studies. Cell. 2019;177(1):26–31. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7380073/
- Bibbins-Domingo K, Helman A. (eds.) Why Diverse Representation in Clinical Research Matters and the Current State of Representation within the Clinical Research Ecosystem. National Academies Press (US); 2022. https://www.ncbi.nlm.nih.gov/books/NBK584396/
- Bibbins-Domingo op. cit.
- Clark LT, op cit.
- Gross AS, Harry AC, Clifton CS, Della Pasqua O. Clinical trial diversity: An opportunity for improved insight into the determinants of variability in drug response. Br J Clin Pharmacol. 2022;88(6):2700-2717. doi:10.1111/bcp.15242
- Clark LT, op.cit.
- Enhancing clinical trial diversity. Deloitte Insights. November 2021 https://www2.deloitte.com/us/en/insights/industry/life-sciences/lack-of-diversity-clinical-trials.html
- Schwartz AL, Alsan M, Morris AA, Halpern SD. Why Diverse Clinical Trial Participation Matters. New England Journal of Medicine. 2023;388(14):1252-1254. doi:https://doi.org/10.1056/nejmp2215609
Are you ready to transform the diversity of your clinical trials?
Use this form to request a consultation with WCG's Diversity, Equity & Inclusion experts.
