Diversity, Equity & Inclusion
Create the world you want to see. WCG’s leading DEI practice has defined policies and processes to engage, educate, enroll, and retain diverse populations in clinical trials.

What if you could achieve clinical trials that accurately reflect the diversity of participant populations?
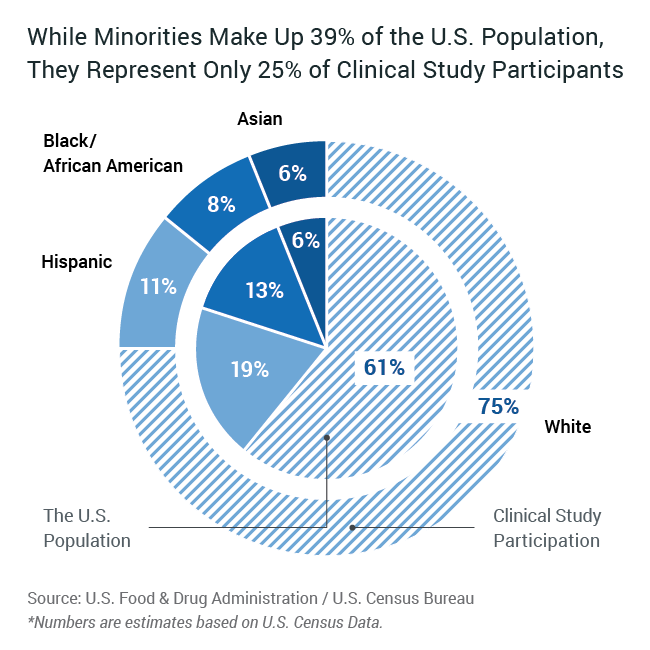
To reduce disparities and advance equity, it’s critical for clinical trials to reflect all communities. Give these participant groups a meaningful “seat at the table” in terms of study design, then discover new ways to engage, educate, enroll, and retain them.
What would appropriate representation look like? You need a cost-effective method that considers therapeutic area, indication and geographic prevalence to drive site selection and recruitment. Leverage our Data Intelligence Platform and deep relationships with patient advocacy and community leaders for more thoughtful, trusted outreach that drives success.
Comprehensive protocols optimize health equity
Our Community Advisory Board method incorporates the participant voice and patient advocacy into your trial planning, design, and conduct – for transformational results:
Streamlined, participant-centric Phase II/III trial designs
Strong portfolio of patient advocacy and community leader relationships
Lower risk of post-approval requirements
Conformance to new FDA guidance for trial diversity


Increase representation and build trusted relationships
With WCG, you gain an expert partner for innovative, turnkey clinical trial solutions. Through our strategic solutions for clinical trial inclusivity and diversity, you’ll become an industry leader in building holistic, sustainable programs based on accurate participant representation and trustworthy outreach.
WCG has been a trusted partner in trial participant protection for over 50 years. Our dedicated patient advocacy group and subject matter experts have deep experience in ensuring clinical trial inclusivity and diversity.
Are you ready to transform the diversity of your clinical trials?
Use this form to request a consultation with WCG’s Diversity, Equity & Inclusion experts.
Explore our insights on Diversity, Equity and Inclusion

Preparing Updates to IRB and Recruitment Processes for the Upcoming FDA DEI Mandate
Blog Posts
FDA Guidance and Revised OMB Guidance on the Collection of Race and Ethnicity Data
Blog Posts