CROs
What are the requirements for submitting protocol deviations?
Blog Posts
Clinical Endpoints
Podcast: The Role of Expert Committees in Clinical Research with guest Jonathan Seltzer, MD, MBA, MA, FACC
Podcasts
Clinical Endpoints
Eliminating the Headache of Global Safety Reporting to Investigators and Ethics Committees
Videos
What elements of Informed Consent must we include when pre-screening?
Blog Posts
Clinical Endpoints
The Role of Expert Committees: An Interview with Dr. Seltzer
Videos
Ethics in Clinical Research
Does the IRB need to review news stories?
Blog Posts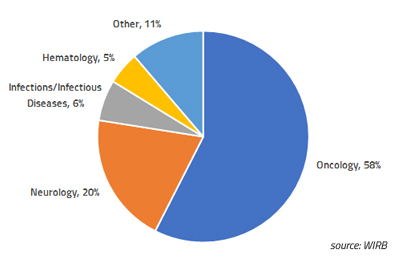
Single-Patient Expanded Access: WIRB experience in 2018
Blog Posts
Limited IRB Review: Are You Prepared for January 21st?
Blog Posts
Site Efficiency
IRBs can make the most of central IRB partnerships
Articles
Clinical Trial Safety