Regulatory Compliance

Ethics in Clinical Research
If My Study Includes Approved Drugs, When Do the Risks of Those Drugs Need to Be Disclosed in the Consent Form?
Blog Posts
Diversity & Inclusion
FDA’s Path Toward Diversity in Clinical Trials: The DEPICT Act and Sponsor Responsibility
Whitepapers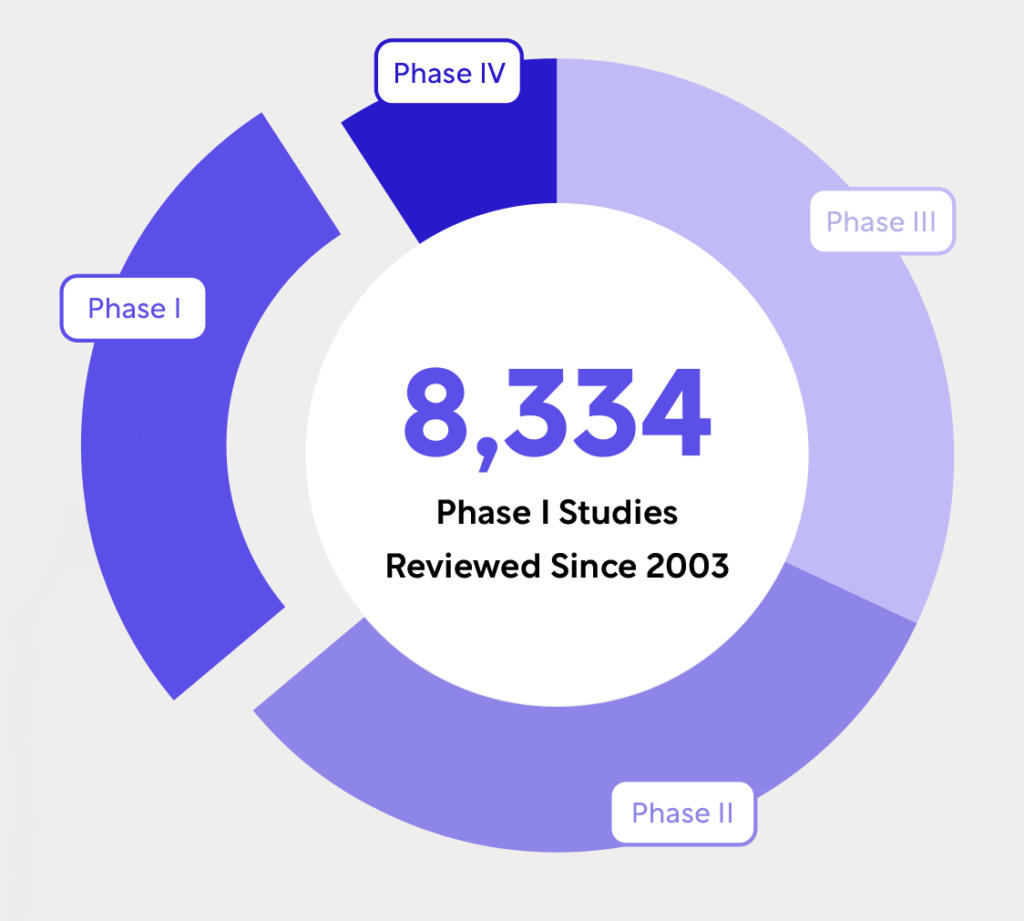
Ethics in Clinical Research
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies
Regulatory Compliance
What You Should Know About FDA Final Informed Consent Guidance
Blog Posts
Clinical Trial Safety
The Nuances of Patient Selection: Why Some Trials Need Eligibility Adjudication
Whitepapers
FDA & ICH
Function over Form: Assessing Different Consent Form Formats
Whitepapers
Cell & Gene Therapy
Why Biotech Sponsors Need Outside Support: IRB, IBC, DMCs and EACs
Whitepapers
Ethics in Clinical Research
Understanding the FDA’s new proposed regulations on human subject research and their impact on your clinical trial plans
Videos
FDA & ICH
Assessing Potential Risks in the Consideration of IND Exemption Criteria
Blog Posts
Ethics in Clinical Research