Patient enrollment goes beyond merely identifying individuals for your next trial. It begins there, but it doesn’t end there. It continues throughout the entire study.
The goal isn’t merely to enroll patients, it’s to successfully complete the study. To that end, we’re going to identify ways sponsors and CROs can help sites optimize enrollment capacity and improve overall study enrollment and retention.
A Flexible, Centralized Strategy
Begin with a centralized study enrollment strategy based on the protocol and targeted population. The centralized plan needs the agility to respond promptly to any site-specific issues and real-world complications.
Enrollment capacity involves all the resources that are needed to complete a trial, such as processes, team expertise, operational efficiency, and technological tools and, of course, time It takes just one flawed process, one obsolete technology, one understaffed area to stall enrollment.
Success requires having the right amount of the right resources in the right combination. Take chart review as an example. Seemingly straightforward, it nevertheless involves numerous elements which require careful attention. Consider:
- Record selection: How are records chosen? Is the EMR easily queried, enabling you to identify the right patient pool before reviewing individual records?
- Evaluation criteria: What are the assessment criteria? Are there elements that will call for physician discretion that shouldn’t be evaluated during chart review?
- Tracking: What are you using to track the chart review to avoid redundant work? Are you using a web portal, a spreadsheet, a sheet of paper or something else?
- Roles and responsibilities: Who conducts the review? Is it a full-time study coordinator or someone who can only dedicate a few minutes a day? Is there a consistent, repeatable process in place?
- Follow up: Once you have a pre-qualified subject, what happens next? Who takes that next action? Does the record get lost in a pile of others, or is there a system to reach out to potential participants?
Chart review is just one example. Most clinical trial processes have components that should be considered individually.
Beyond Inclusion/Exclusion Criteria: Participants as Individuals
Turning a patient into a participant involves much more than the inclusion/exclusion criteria. Understanding the real person behind the I/E criteria helps build vital relationships—relationships that support enrollment and retention. Being a study participant means the patient has volunteered their time and their body for the study. Will that person be willing to take part in a 24-month study? Are they willing to commit to regular visits?
It’s crucial to understand each participant’s motivations, not just at the beginning but throughout their journey through the study. What are their reasons for joining this study? What barriers might prevent them from participating or completing it?
By focusing on the unique needs and motivations of each participant, we can build trust and foster a more patient-centric study environment. This is especially true for unknown patients.
When we talk about a “known” patient, we’re talking about someone who already has a relationship with the site and/or the PI. There’s already a level of trust. But what about “unknown” patients—patients with no prior relationship? It’s important to understand them, build that trust to work with the clinical study site team, to motivate them not only to enroll, but also to stay in the study. It begins with that first encounter: That first impression – be it a phone call, a flyer, a website visit, an online ad – needs to be a positive one.
Diagnose the Problem, Then Act
The enrollment process will rarely go exactly as planned. That’s why it’s important to identify and address challenges early, tailoring interventions to each site’s needs and limitations
Begin by examining all existing processes. Pinpoint the bottlenecks. For example, if enrollment is lagging, the site may need additional support for recruitment and enrollment. In other cases, data entry and query resolution are falling behind, and the site needs support there.Providing data entry support, in turn, may create additional bandwidth to continue focusing on recruitment efforts.
Focus on implementing solutions that enhance the site’s capacity without compromising efficiency.
Here, it helps to think about the study lifecycle. Themore time you have, the more options you have. Efficiency tends to falter when timelines shorten. So, the more you can do in advance and plan and prepare, the better.
Ask yourself: Do the tools and resources we’re providing increase the site’s bandwidth, or do they add extra steps? You want to increase the site’s capacity, not its burden. For example, if a study requires a patient to be newly diagnosed, a retrospective chart review won’t prove very helpful.
Think about this: Are you truly enhancing the site’s capacity to enroll more patients? Or are you imposing additional processes that could slow enrollment and diminish the site’s interest in your study –or in ever working with you again?
Put it in Practice
Learning from patients and sites and sharing best practices among different sites can be valuable.
Study coordinator meetings provide the opportunity to elicit best practices that other sites can adopt. It’s also an opportunity to learn what isn’t working.
In trials involving multiple vendors, coordination and oversight are crucial. Aligning vendors’ efforts and ensuring a shared vision for recruitment success requires proactive management and collaboration.
Setting benchmarks and key performance indicators (KPIs) is essential for assessing recruitment success. However, use them as evaluation tools, not as penalties. Foster an environment where everyone feels safe to flag problems early, identify them and work towards a solution without fear of retribution. The process never stops: Evaluate. Plan. Deploy. Assess. Adjust. Repeat. (See figure 1.)
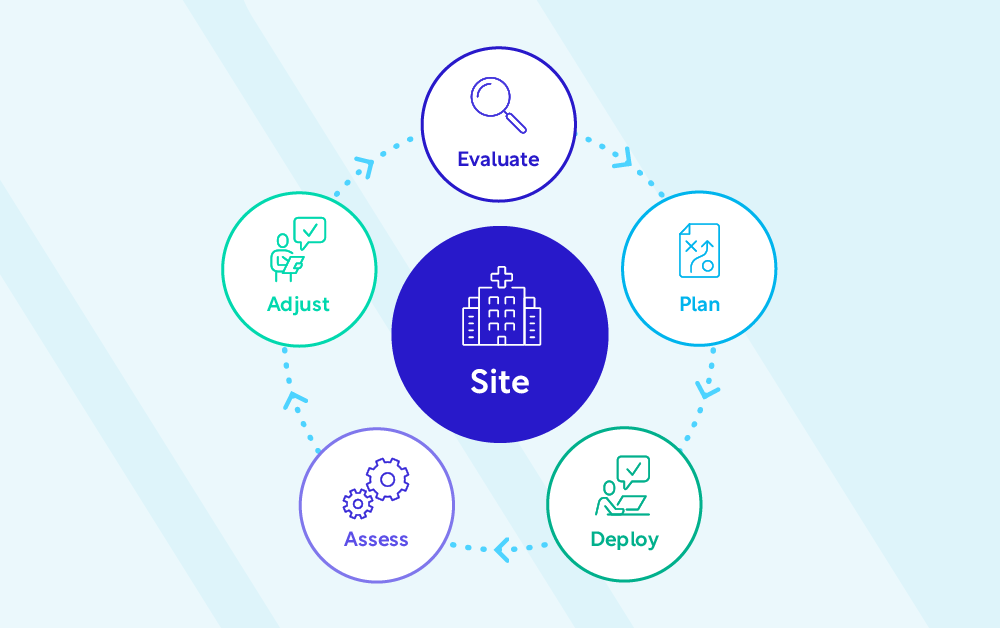
It bears repeating: Change is inevitable, and your plans will require adjustments. That’s why enhancing clinical trial enrollment requires a comprehensive and flexible approach that considers the site’s capacity, patient motivations, and ongoing adjustments based on real-world challenges.
When we all come together and take this approach of being flexible and adapting to change, we can solve most problems.
Your Enrollment Challenges. Our Expertise. Take charge of your study’s outcome.
Connect with our team of experts to discuss your studies and how we can quickly meet patient enrollment milestones.
Find out how many months our Participant Enrollment services can cut from your enrollment timelines
Complete the form to schedule a consultation with WCG. We’ll share benchmark data, analyze your results, and share some of the common practices of top performers.