Hematology/Oncology Solutions
Optimizing the modern Oncology clinical trial process
Connecting your hematology/oncology programs with tailored solutions to support your success.

A specialized, experienced partner to help you navigate the path to success in hematology/oncology clinical trials
As oncology drug development is moving from drug-centered to patient-centered, WCG can help navigate the changing landscape so you can make decisions quickly. WCG brings a new approach to clinical development, leading with innovative, tailored solutions to help you focus on the patient, manage trial complexity, and increase predictability and speed.
Through our strategic approach to hematology and oncology program development, WCG can help you rethink old challenges and surround your trials with a proven suite of capabilities that enable you to reach milestones faster, deliver programs within budget, and bring game-changing therapies to patients sooner.
The WCG Difference in Oncology
Specialized Team
Scientific, medical, and operational experts to help plan and execute hematology/oncology programs
Agility and Flexibility
The solutions you need for your specific studies – at the right time and in the right order
Access to Hematology/Oncology Sites
Our established relationships with sites open doors for your hematology/oncology studies
A Suite of Solutions to Accelerate Oncology Studies
IRB Review
IBC Review
Total Feasibility
Patient Identification
Patient Recruitment & Retention
Imaging Core Lab
Statistical Consulting
Training
Site Augmentation Solutions
Our Hematology and Oncology Expertise
Site-based Services to Accelerate Oncology Study Timelines
WCG provides global on-site services tailored to meet the challenges of patient recruitment for oncology studies, helping you meet or beat your enrollment timelines.
By augmenting existing site resources, you also assist busy oncology practices and sites with the higher data entry demands, increasing the speed and quality of study data entry.
Our experience includes international support for more than 100 oncology studies, with multi-specialty areas including liver, bladder, prostate, colorectal, bone, ocular and various solid and hematologic cancers.

Accelerate the performance of your oncology trial
Move your oncology study forward faster with expert guidance from top industry leaders, vital insights from the industry’s leading data intelligence platform, global on-site services, and more. Start your journey with WCG by scheduling a complimentary meeting with one of our oncology experts.
Recent insights from our Oncology trial experts

The Ethical and Safety Considerations of Accelerating Oncology Trials
Podcasts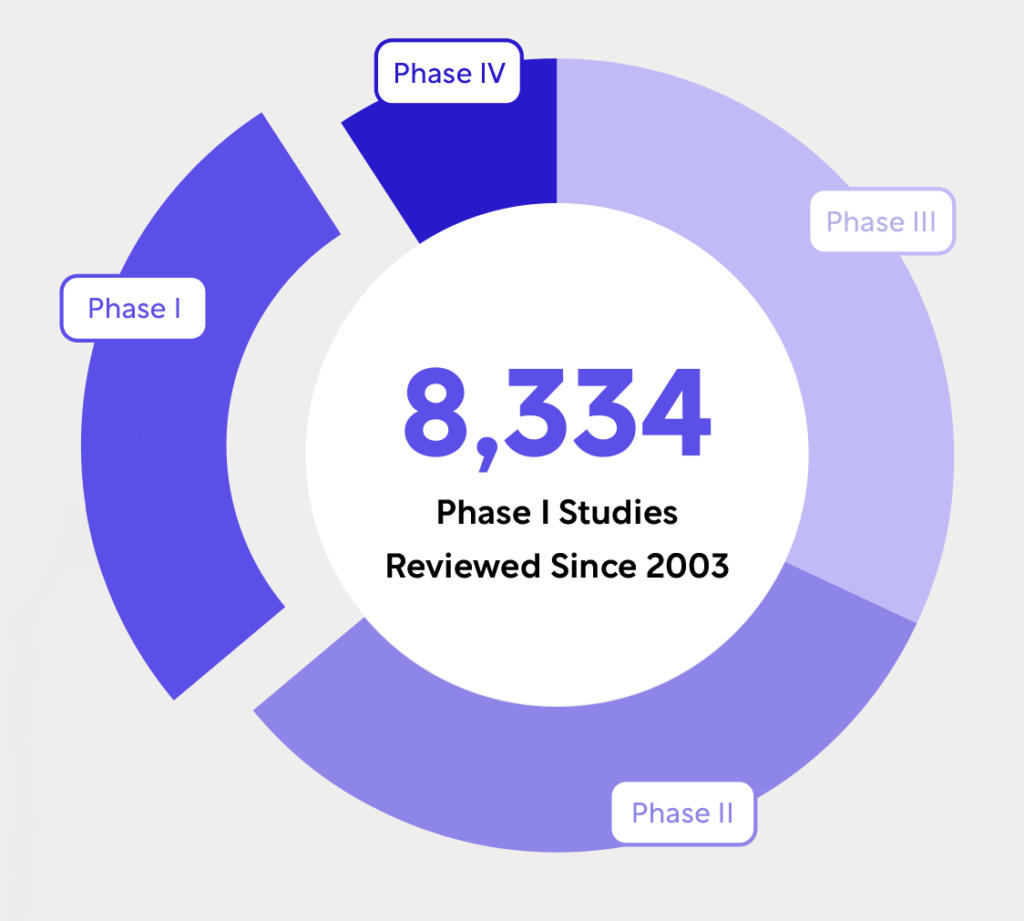
WCG’s Unmatched Experience in Early Phase Hematology and Oncology
Case Studies