The Great Resignation. The Big Quit. The Great Reshuffle. This phenomenon began in early 2021 as an economic trend where employees resigned from their jobs en masse. The exodus was rooted in workforce issues, including wage stagnation amidst a rising cost of living, long-lasting job dissatisfaction, plus pandemic-related safety concerns, and remote work policies. Today, The Great Resignation can be viewed through a new lens, courtesy of Forbes: The Great Re-Engagement. This two-part blog looks at how clinical research sites have been affected and how innovators are forging new solutions, and in this blog, we will dive into the current pain points and obstacles sites are facing.
Study Starts
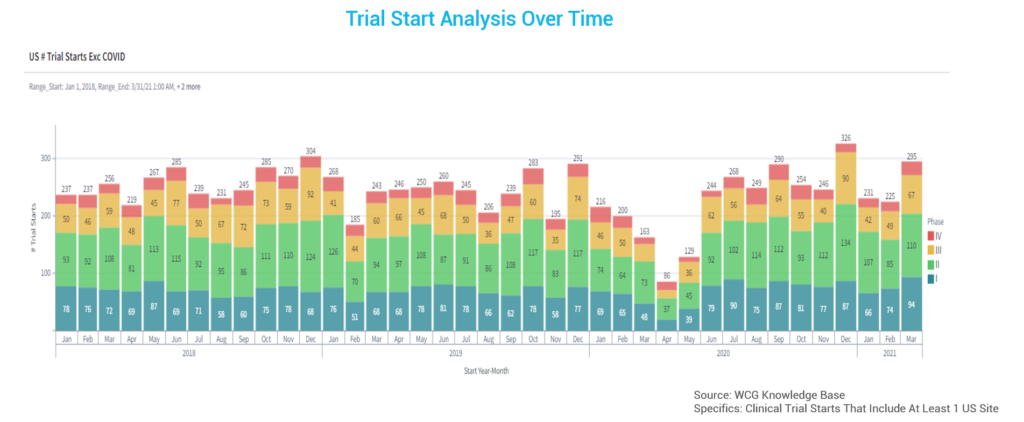
Let’s look at Q1 of 2022 within the clinical trial landscape. Q1 was the busiest quarter for study starts across all therapeutic areas (excluding COVID trials). Notably, this quarter included quite a few Phase III trials, in addition to many trials whose timelines are being pushed out, possibly due to a lack of resources.
Resignation Rates
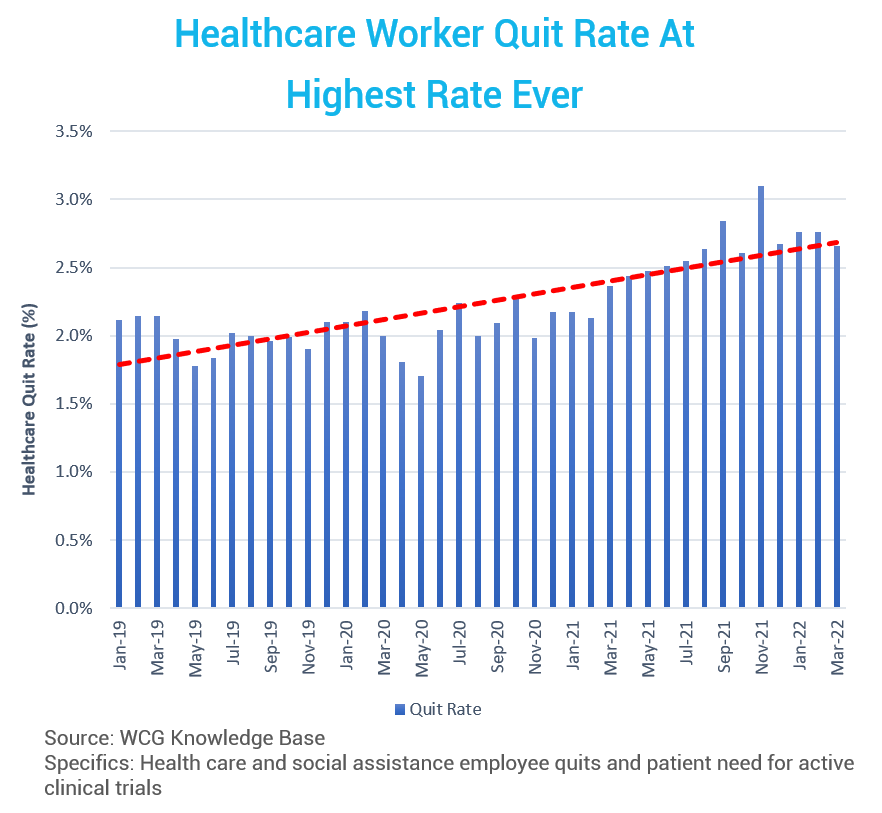
Today, we are seeing the highest resignation rates in health care ever, including people moving to new positions or retiring.
The resignation rate continues to trend upward (red line). What does this mean for clinical research? It means we have more study starts than ever, combined with a higher healthcare resignation rate – meaning fewer resources – than ever.
Patient Participation
Next, let’s look at subject participation in clinical trials. While the resignation rate of healthcare workers continues to increase, nearing 3.0%, the total number of participants needed for clinical trials (red line) is also increasing.
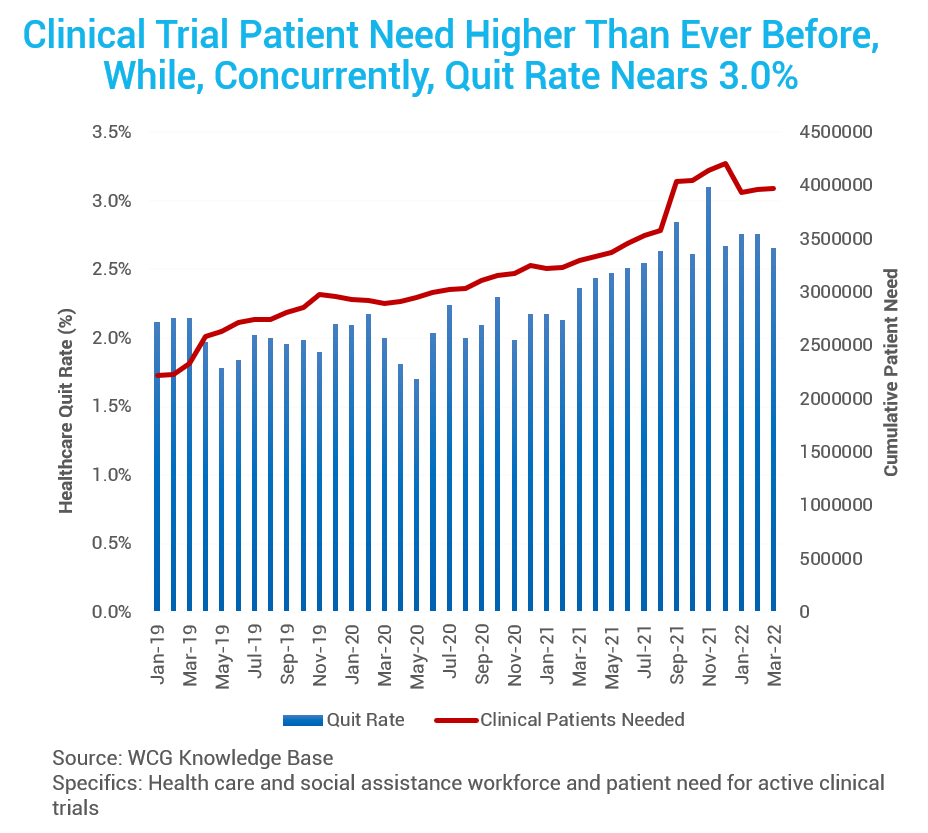
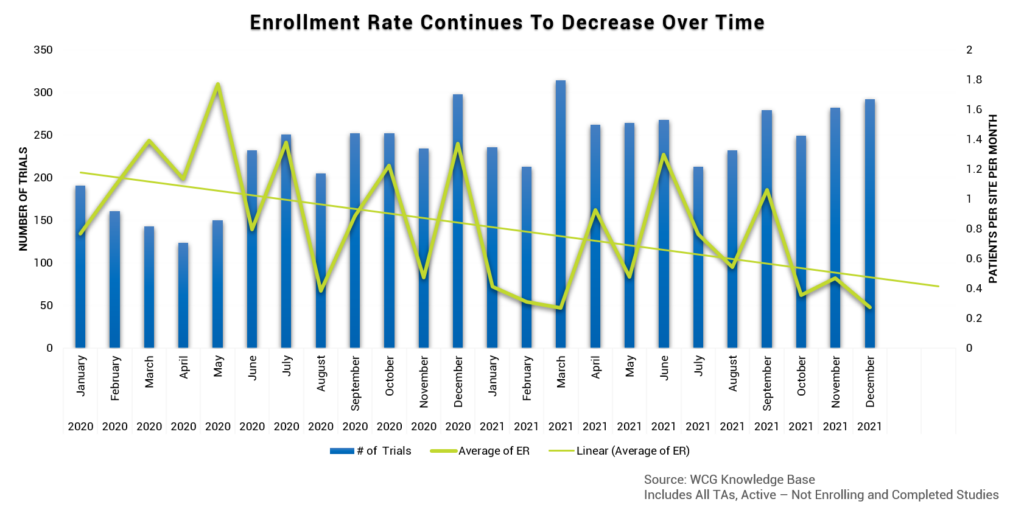
At the same time, patient enrollment rates are declining. We are seeing a 50% decrease in patients per site per month across all therapeutic areas, especially in oncology. Respiratory numbers are also relatively low, no doubt impacted by COVID. The bottom line? The culmination of a huge resignation rate + more study starts than ever + an increase in participants needed + a decrease in study enrollments = tremendous pressure on research sites.
Pain Points
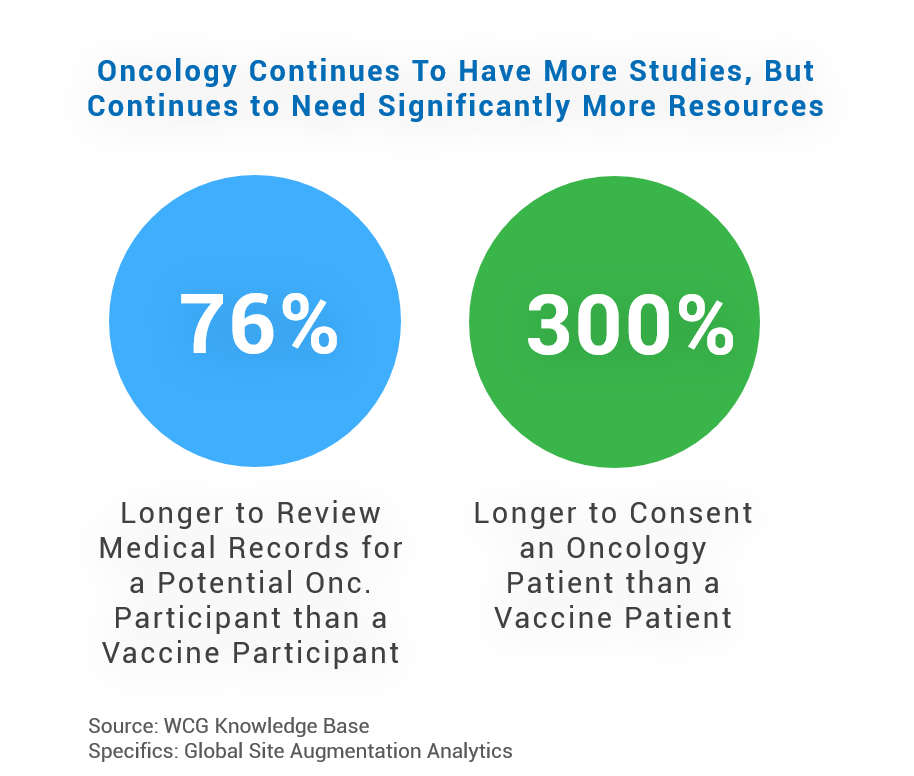
Since half of the Q1 2022 study starts are in oncology, this area keenly feels staffing challenges. Note that oncology trials also require the most resources across every phase – including study coordinators and research nurses. It takes 76% longer to review medical records for oncology subjects than for vaccine subjects. Teams may have to review three or more pages of inclusion/exclusion criteria within an oncology protocol to determine a candidate’s potential. This population also takes 3x longer to consent than vaccine patients. These factors intensify timeline pressures and site burdens.
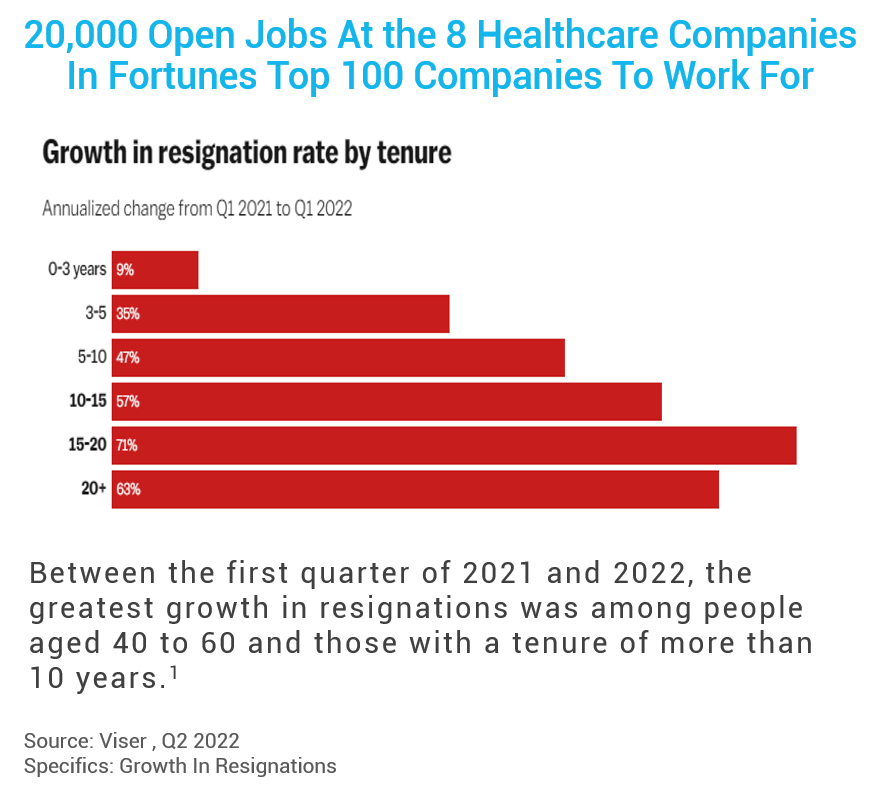
Between Q1 2021 and 2022, the most significant growth in resignation was among people aged 40-60 and those with higher tenure. Losing healthcare workers with 15-20 years of experience results in a tremendous loss of institutional and clinical research knowledge, a phenomenon dubbed “brain drain”. An employee with six months of experience does not have the same capabilities as a 10-year veteran, so there is a trickle-down effect on employee education with a steep learning curve. Some sites report it takes up to a full year for new clinical researchers to develop the skill level of those vacating the positions. New team members must essentially train for two jobs – learning research plus the therapeutic area.
As a result, sites report a variety of pain points and obstacles to success:
- Putting a hold on opening new studies
- Being at half-staff for study coordinators
- Having an 80-100% turnover in regulatory staff
- Losing 50% of their experienced research team
- Having 10-12 studies onboarding without a CRC available
Read the second part of this blog, where we will focus on strategies and ideas to combat these struggles and more!
Interested in learning more about how WCG can help your site navigate the changing clinical trial landscape? Contact us today to chat with our site augmentation experts.
