The Great Resignation. The Big Quit. The Great Reshuffle. This phenomenon began in early 2021 as an economic trend where employees resigned from their jobs en masse. The exodus was rooted in workforce issues, including wage stagnation amidst a rising cost of living, long-lasting job dissatisfaction, plus pandemic-related safety concerns, and remote work policies. Today, The Great Resignation can be viewed through a news lens, courtesy of Forbes: The Great Re-Engagement. This two-part blog looks at how clinical research sites have been affected and how innovators are forging new solutions. In part one, we dove into the current pain points and obstacles sites are facing, and in part two, we will focus on strategies and ideas to combat these struggles.
Site Augmentation
What are some solutions to the challenges of The Great Resignation? Obviously, managers are prioritizing. If you are operating at half-staff, where do you place your resources? Sponsors and sites leveraging WCG Site Augmentation services report feeling less burden because they were able to outsource functions to a proven provider. Here are some of their comments:

Regulatory function is one area sites are outsourcing – from study startup to initiating proper documents to submitting to the IRB. However, not every site needs the same support; it varies from site to site, study to study, and indication to indication. This variation means that support must be adaptable.
Flexible Services
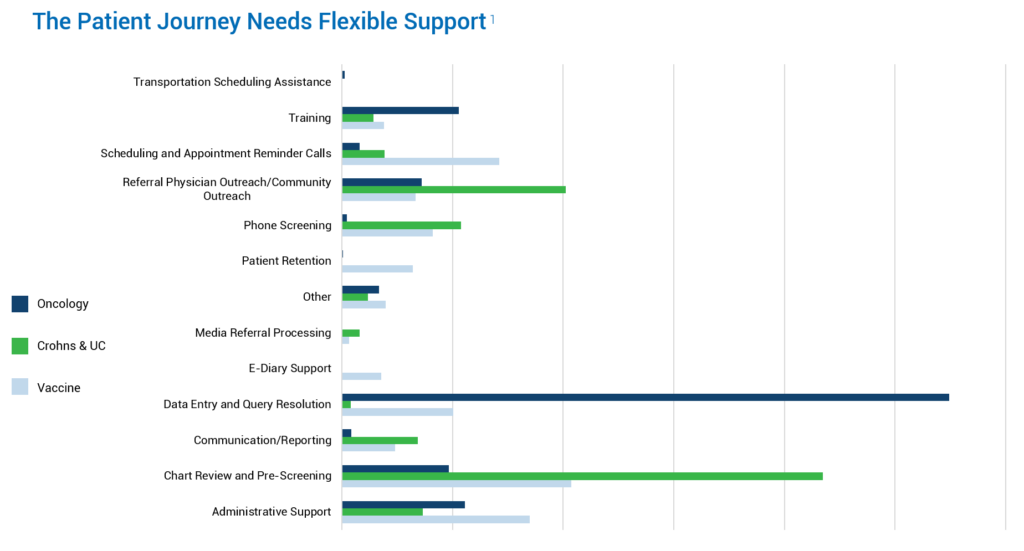
There are striking differences in site support needs based on the type of trials conducted. The most significant variation is in data entry and query resolution, which can cause a massive burden for oncology sites. Also, consider study complexity – the schedule of events, sheer number of visits, and all the testing and diaries inherent to oncology studies.
We’re back to the issue of prioritization. Do you put your time into enrolling patients, only to see the data suffer? Or do you put your time into the data, only to see recruitment and enrollment suffer? Especially in oncology studies, sites want to maintain that patient contact. Along with the time-consuming consenting process, there is a compassion element, and the patient-site bond is strong. Therefore, WCG Site Augmentation is often utilized for back-end functions, accelerating site data entry and query resolution. This way, sites can continue their focus on enrollment while meeting their data submission timelines.
Another function for consideration is chart review and pre-screening, which is a prominent research function with the Crohn’s and ulcerative colitis indication. Finding those patients can place a strain on on-site resources, so augmenting helps accelerate the review of charts and pre-screening of patients while the site team handles consent and maintains patient relationships. For the vaccine area, we see the entire gamut; there is certainly an administrative piece with chart review. The need is driven by several factors, including the experience level of new team members and the personnel gaps. For some sites, WCG Site Augmentation can provide an experienced resource to assist with training – relieving that burden and allowing the study team to conduct the trial.
New Ideas
As an industry, we are rethinking how we conduct clinical research:
- We need to match the right people with the right tasks at the right times and then fill in the gaps.
- We must look at the workflows and re-visit what can be done on-site vs. remotely. Examples may include remote clinical research coordinators and regulatory coordinators.
- We can gain efficiencies by seamlessly augmenting the research team and providing needed support. Examples include IRB submissions, enrollment screening, participant retention, and data entry.
- Training is another area where we can apply new ways of doing things while offloading the site staff. Examples include training for GCP, study protocols, and general competencies.
- Technology is a significant contributor to site challenges. Sites report having as many as five different technology platforms per study; multiplying by the number of studies, a new CRC may have to learn 30, 40, or 50 different technology platforms.
- Sponsors are willing to help sites by providing support. Increasing communication with sponsors and developing the sponsor-site relationship can help relieve bandwidth issues.
- Finally, pay attention to the onboarding process. Make sure that as existing and new staff members move through their days, they still connect as a team. All team members should enjoy their work and feel appreciated.
Additional Resources


Interested in learning more about how WCG can help your site navigate the changing clinical trial landscape? Fill out the form below to chat with our site augmentation experts.
