Study Insight Analytics
Detect. Assess. Intervene.
WCG’s Study Insight Analytics combines statistical process control and other analytical techniques with real-time human expertise. This approach provides insights and actionable recommendations, allowing sponsors to address data-quality issues and protect study outcomes.
For all trials, particularly those with subjective measures, the sponsor or contract research organization needs a way to identify and mitigate data-quality risks. Now they can.
With our platform, sponsors increase the likelihood of a positive outcome while meeting scientific and regulatory expectations for data quality.

Statistically aberrant signals detected
Clinically relevant signals evaluated and prioritized
Sites monitored worldwide
Identify and respond to study issues in-flight
Throughout the course of a trial, our Study Insight Analytics monitor selected metrics. This allows it to identify statistically significant and clinically relevant aberrant signals in real-time. The project team can then swiftly address threats even while the trial is running and blinded.


See retrospective analyses of completed trials
From protocol design to study conduct, your past trials contain important lessons. Retrospective analysis unlocks these lessons, all but eliminating guesswork for future trials. We can perform retrospective analyses of your past trials to identify patterns and gain insights into what went right–and what didn’t. You can learn from your stunning successes and your most painful failures.
Our platform combines study analytics, retrospective analysis, and clinical expertise to inform your next phase of development and increase the likelihood of clinical trial success.
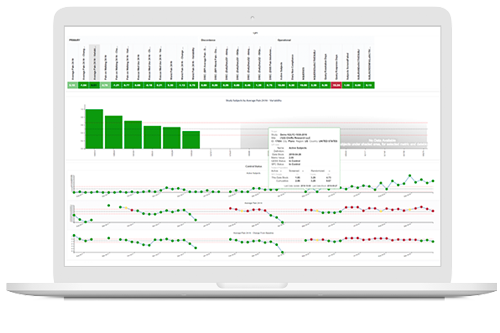
Detect aberrancies early
Capture statistically significant signals of aberrant performance at the participant, site and study levels for assessment, intervention and follow-up.
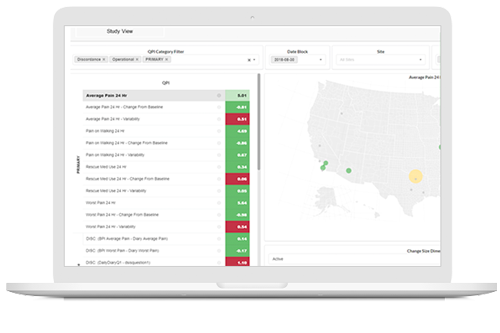
Turn insights into interventions
Identify threats to the outcome of the study before they do lasting damage, and ensure interventions are deployed to address data-quality threats as they are detected.
To see a demo of our Study Insight Analytics platform, request a consultation today.
Learn how our Study Insight Analytics can pinpoint and analyze anomalies in your clinical trial data collected remotely or on site. Schedule a consult today.