Clinical Research Trends & Insights for 2022
In our 2022 preview, 19 experts from WCG share the important shifts, trends, regulations, and priorities that will inform clinical trial development this year and beyond.
Once again, as the end of a calendar year approaches, we start looking forward to the next one. As we’ve done for several years now, WCG has asked our leadership and subject matter experts to share their thoughts about what trends, changes, and innovations they are looking forward to in the coming year.
2021 brought us a year of relative recovery in the clinical research world; though we’re still in the midst of a pandemic, the research slowdown of 2020 is in our rear view, and there were more new clinical trial starts in late 2021 than ever before. We’ve adjusted to new ways of working and new ways to design and conduct our clinical trials.
Like all of you, we look forward to what the new year will bring, and to the developments that will help us to conduct ethical, scientifically-rigorous research and continue to advance new therapies.
SECTIONS:
Regulations and Guidance:
Interpretations and Expected Updates
Perspectives from Clinical Sites
Data, Technology and Operations
Clinical Safety and Study Endpoints
Scientific Considerations:
Advances in Gene Therapy and Oncology
The Patient Voice:
Advocacy and Awareness of
Under-Represented Populations
Watch our 2022 Trends & Insights Webinar



Hear our online panel of clinical trial experts as they discuss the future of clinical trials in this on-demand webinar:
Regulations and Guidance:
Interpretations and Expected Updates
Anticipated SACHRP Recommendations
The U.S. Department of Health and Human Services’ Secretary’s Advisory Committee for Human Research Protections (SACHRP) continues to advise the Office of Human Research Protections on a number of issues that are timely and may result in recommendations in 2022 that could potentially influence regulatory interpretation by OHRP and FDA.
Topics with expected recommendations in 2022 include the use of artificial intelligence (AI) in research, risks to third parties in research and the “rules of engagement” that OHRP uses to determine when an institution participating in research becomes regulated.
AI has become a hot topic in several applications including research. SACHRP will be looking at the ethical issues that are generally raised using AI in research. They will make recommendations regarding the role of the IRB when reviewing research with AI and the role of regulators in overseeing AI research.
The IRB regulations require that IRBs evaluate risks to subjects in relation to the expected benefits of the research to subjects or others. However, we know that individuals who are not research participants may be exposed to risk. SACHRP will be considering whether there is a role for IRBs in identifying those risks and working with research teams to mitigate them. This raises concerns about IRB mission creep and what criteria IRBs would use to assess these risks.
Finally, the issue of engagement in research has always lacked clarity, particularly with respect to research projects that have multiple organizations playing different roles. Is an institution that is serving as a data coordinating center subject to the same IRB requirements as the sites that are implementing the clinical research that produces the data? Existing OHRP guidance provides some relief. However, a large gray area remains. SACHRP will be examining the current understanding of engagement and possibly make recommendations for refining that understanding with an eye towards reducing regulatory burden that does not add to human subject protection.

David Borasky, MPH, CIP
Vice President, IRB Compliance
WCG IRB
Global Privacy Regulations
In 2021, we saw existing privacy regulations continue to evolve, while new privacy regulations were in the process of being implemented in many countries and states. These regulations need to be balanced with the requirement to maintain appropriate documentation of clinical trials. Many of these regulations have provisions excepting clinical research, but they vary in scope and in specific wording.
In addition, there are many unique laws at a local level. Transferring data among countries remains challenging, particularly from European Union countries to other parts of the world. For instance, in France the MR-001 requires explicit permission from the French privacy agency, CNIL, for the external transfer of clinical data, including identifiable images, even though the GDPR does not have such a requirement.
In 2022, we will see data localization requirements in certain countries, including Russia and China. Both privacy and clinical research are public goods, and the balancing of these goods will continue to be a priority and an administrative challenge.

David Forster, JD, MA, CIP
Chief Compliance Officer
WCG
Increasing Use of RWD/RWE in Drug Development
There is an increased interest in the use of real-world evidence (RWE) and data (RWD) to support the continuum of evidence generation for regulatory decision-making for drug and biological products, both in the EU1 and U.S.2
The very recent publication by the FDA of its new draft guideline in this space is worth noticing.3 According to the FDA, the use of computers, mobile devices, wearables and other biosensors to gather and store huge amounts of health-related data has been rapidly accelerating. These data hold potential to better design and conduct of clinical trials and studies in the healthcare setting to answer questions previously thought infeasible.3
In addition, with the development of sophisticated new analytical capabilities, we are able to better analyze these data and apply the results of our analyses to medical product development, approval3 and reimbursement. It is expected, for instance, that RWD should enable the generation of additional evidence post-launch, inform dynamic price-setting in relation to the value of medicines and may optimize appropriate use in daily practice.
However, several challenges emerge, such as how to manage expectations about the use of such data, how to better understand their usefulness and their pitfalls throughout an entire medicine’s lifecycle (and not just post-launch) and how to encourage their optimal use.
References:
- “Get Real Institute” website homepage: https://www.imi-getreal.eu/
- Sherman RE, et al. Real World Evidence- What is it, and What Can it Tell Us? N Engl J Med 2016; 375:2293-2297
- Real-World Data: Assessing Electronic Health Records and Medical Claims Data To Support Regulatory Decision-Making for Drug and Biological Products: Guidance for Industry. FDA Draft Guidance, September 2021. Accessed at: https://www.fda.gov/media/152503/download

Luca Pani, MD
Vice President, Regulatory Strategy and Market Access Innovation
WCG VeraSci
New ICH Guidance driving Quality by Design (QbD)
In 2022, I predict that ICH-E8(R1) and updates anticipated in ICH-E6(R3) will drive industry exploration and increased adoption of quality by design (QbD), risk-based quality management (RBQM) and centralized monitoring (CM) approaches. Sponsors will embrace QbD principles to ensure that quality is designed into study protocols and processes, including the perspective of patients living with the condition being investigated. Additionally, the use of critical-to-quality factors described in ICH-E8(R1) will support implementation of RBQM and CM approaches outlined in the ICH-E6 (R2) and (R3) draft addendums.
To support successful implementation of QbD, RBQM and CM programs, organizations will need to address two important areas:
- access to operational data to support RBQM and CM data analytics; and
- a shortage of staff trained to interpret and act on the data.
These challenges can be addressed through the adoption of industry-based performance and quality metric standards that improve the quality and consistency of the data available to data analytic programs. Additionally, risk management and root cause analysis training programs — developed specifically for clinical research staff — can be deployed to close the workforce skills gap.
I believe that 2022 will be the year that the industry moves from piloting to fully embracing QbD, RBQM and CM approaches by designing less burdensome trials and using data to identify when human intervention is needed to determine whether patient safety and/or data integrity issues are occurring and to take timely action before they impact the integrity of the research.

Linda B. Sullivan, MBA
Executive Director
WCG MCC
Perspectives from Clinical Sites
Changes in the Infrastructure of Clinical Research Sites
I believe the impact from the pandemic’s disruption of healthcare research operations in 2020 and 2021 will start to manifest in greater emergence of alternative models for site participation in the coming year. Traditional healthcare research entities and dedicated research sites are currently struggling with staffing solutions to meet the needs of trials underway and new protocols coming from sponsors. Technology solutions such as clinical trial management systems, eRegulatory systems, automatic data capture, etc. have failed to yield substantial improvements in terms of labor reduction per study for sites participating in clinical research. Capturing new sponsored clinical trial opportunities by adding research coordinators is not a viable solution in the current labor climate. This creates an environment for new players or for redesigned models from previously underutilized players to gain (increased) business in sponsored clinical trials.
New models of research-site organization that promise improvements in efficiency, data quality and increased enrollment should begin to get a greater foothold in the industry in 2022.
Focused site networks, community-based provider consortiums, joint ventures that combine resources from former healthcare competitors and the expansion of clinical trial research conducted by nontraditional entities such as Optum/United Health Care or Aetna/CVS have the ability to gain greater research work in coming years. Furthermore, research sponsors’ focus on conducting U.S. clinical trials using fewer sites, which reliably deliver greater enrollments, sets the table for breaking traditional relationships in favor of higher performance.

Geoffrey Schick, MBA, CHRC
Director, Strategic Site Partnerships
WCG
Better Support for Clinical Research Sites
In 2022, we’ll continue to see trends of high turnover, increased protocol complexity and the use of disparate technologies all impacting research sites the most. But, because of those challenges…
…our industry will develop a new approach to build better networks that support research as a care option, specifically in oncology and CNS. Sponsors are keen to reach new patient populations, and sites want to bring new therapies to their community.
These community-based clinics and hospitals see the highest volume of patients, while having the lowest clinical trial opportunities. It is a moment of confluence to empower new physicians to join research, reach a more balanced participant demographic and break out of the decades-old enrollment challenges. With patient-centric policies and sponsors’ willingness to innovate, we’ll continue to make an important shift for clinical trial access and equity.
We’ll also see experienced research sites push for better budgets and clinical trial agreement terms to ensure the sustainability of their programs and resourcing models. In 2021, sites built creative resource models to help them retain staff, and their costs went up. Sponsors and CROs will adapt to that cost increase to maintain access to these sites and their patients/participants. Sites may walk away from low study budgets and remain selective to only work with sponsors and CROs willing to adjust.
Sites will continue to adopt new business models to run more efficiently, retain key staff and grow their sponsored research volume. Based on a December 2021 survey, we know health care executives1 value research to provide premium care and to remain competitive in their respective markets. They will continue to prioritize access to clinical trials to improve patient outcomes.
References:
- Accessing Tomorrow’s Treatments Today – Defining the Value of Clinical Research in a Health System 2021, survey conducted by Healthcare Dive and WCG. (Data being prepared for publication as of November 2021)

Meg Trost
Vice President, Study Start-Up and Administration
WCG
Increasing the Public Visibility of Clinical Trials
As biopharma grapple with the facts of increasing drug development costs and decreasing returns, study teams need to identify innovative ways to conduct trials more efficiently, requiring less manual brute force and engaging patients in a powerful way to participate. Most of the industry continues to conduct trials the same way while expecting a different outcome.
A view to the future starts with a desire to streamline processes and integrate disparate data, thereby providing transparency into the study’s conduct. Utilizing the collective team’s experience will be required rather than continuing to conduct the trial in a variety of operational silos.
As COVID-19 vaccines and treatments continue to be a nightly story on the global news channels, the public will continue to discover how new, evidence-based therapies are developed and identify tools for learning about their own health and continued health advancements.
The clinical research industry will benefit from further educating the public about the rigor of the trial process and the quality of the experience. It will require more participants to tell their stories, allowing more visibility into the overall drug development process.
Clinical providers will start to speak the language of clinical research, and hospital networks will continue to connect to systems, allowing clinical trials to become more of a care option for patients. Organizations will increasingly tap into a shared network where doctors and patients will receive eligibility alerts in their EHR interface, and patient collaborative care teams will increasingly include trial staff.

Jill Johnston
President, Study Planning and Site Optimization
WCG
Data, Technology and Operations
Rethinking Data Analysis
In 2022, I believe that we will see an exponential increase in the use of human-enabled artificial intelligence (AI), demonstrating its essential contribution to the success of clinical trials.
At its core, data science in clinical trials focuses on detecting trends and finding outliers (signals); but to be fair, that’s the easy part. The problem is that it is very difficult to tell if those signals of interest are clinically meaningful without deep clinical knowledge.
The increasing deluge of clinical data enabled by technological advances, such as devices and wearables, is forcing the industry to rethink not just how we think about data but also who is involved in the analysis.
Advances in research and technology allow us to collect as much data as we want, often without knowing if they will be useful or not. This progress brings new challenges. We no longer have the luxury of looking at every data point and must find ways to prioritize our focus to clinically relevant areas.
In this ever-evolving field, there are few unchanging rules, and what matters at every trial can vary widely. Clinical science and data science must partner closely to gain insights from data and act accordingly. AI and machine learning cannot give us the answers to clinical questions in a vacuum; we need to guide the analysis with expert clinical knowledge. Just because something is mathematically significant, does not mean that it is clinically relevant. We can use data science to guide where to look and we can use technology to make the explorations easier, but we need subject matter experts (SMEs) to convert data into insights. The expertise of these SMEs will become increasingly sought-after in the coming year and beyond.
The good news is that human-enabled AI, when used properly, is very powerful and is already becoming indispensable. It allows us to have a real and measurable impact on the success of clinical trials and we are just getting started on this collaborative journey.

Arturo J. Morales, PhD
Vice President, Data and Technology Solutions
WCG
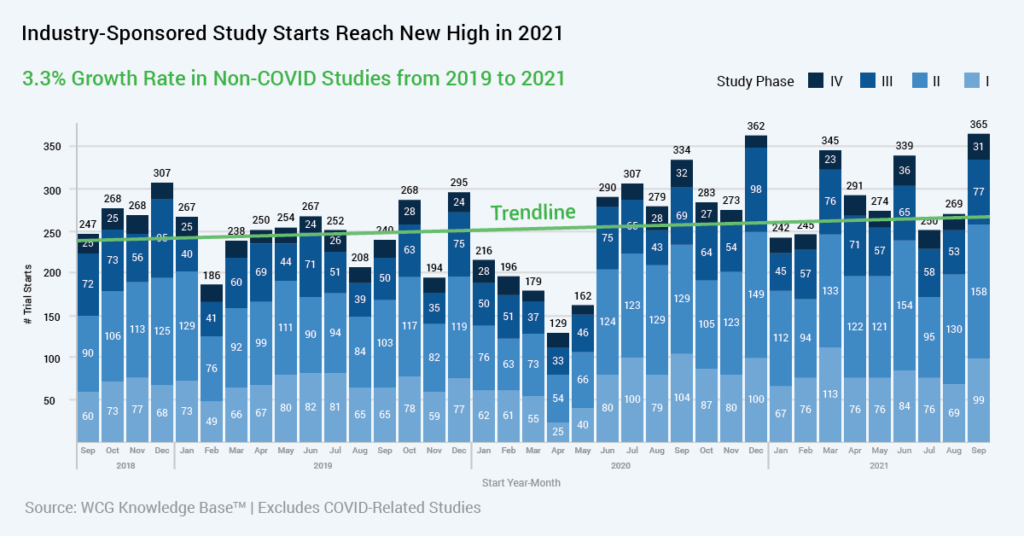
Increasing Remote Data Collection
The clinical trial landscape has continued to evolve in 2021 as we begin to move past the COVID-19 pandemic. New trial starts in 2021 are up from 2020.1 Protocol complexity has also increased significantly. In phase 2/3 trials, data show a 69 percent increase in the number of procedures, and three times more data points being collected (compared to 2009-2012).2
As an industry, during this time, we have learned to develop new methods for our trials that allow for decentralized assessments and that experiment further with new data- capture techniques, including wearables. These efforts not only have allowed for protocols to continue to run during COVID, they have also impacted patient and site burden.
Innovation has significantly enhanced the capabilities of wearable devices, which has resulted in the increased use of the technology in clinical trials. A search of the current ClinicalTrials.gov database shows more than a thousand clinical studies that are completed or ongoing, which include the words “wearable” in the study title.3 Wearables have been used across multiple therapeutic areas including oncology, neuroscience and the study of respiratory and metabolic disorders; and I believe we will see that broaden in 2022 and beyond as innovation continues to drive solutions.
While there are some risks with wearables (data privacy, data validation, logistics), there are many benefits.
In 2022, we will continue to greatly reduce patient and site burden by the remote collection of data. Sponsors will also have near real-time access to data to be able to make critical trial decisions faster. Patient enrollment and retention may see a significant benefit from using these technologies, and costs for data collection should be driven lower.
As clinical trials become more decentralized and innovation continues to drive us toward new methods of data collection and assessment of endpoints, I believe the benefits of wearables will outweigh the risks and they will play an ever-increasing role in the clinical trial landscape.
References:
- Internal Data from the WCG Knowledge Base
- Impact Report: Analysis and Insight into Critical Drug Development Issues. Tufts Center for the Study of Drug Development. Volume 23, Number 1. January/February 2021.
- ClinicalTrials.gov search, keyword “wearable”, any enrollment stage; performed on 1 December 2021.

Michael F. Cioffi
Senior Vice President, Clinical Solutions and Strategic Partnerships
WCG
2022: A Year of Transition
The year 2022 will be our industry’s “transition year” for moving toward more agile approaches and a next-generation clinical trial execution model. Companies will be more acutely aware of the need for greater interconnectedness of the clinical trial ecosystem.
Decentralized Trials (DCT) positively impact patient retention and the diversity of patients participating in clinical trials. Quality with DCT is enhanced rather than compromised.
However, challenges with seamlessly implementing DCT activities and technologies exist and will continue into 2022 with many organizations experiencing operation and process integration as their most significant challenge.
Pharma and biotech sponsors will double down on efforts to improve their approaches to innovation adoption. Collaboratives will continue to play an important role in driving change and moving the industry toward common approaches. Perceived risks with the use of specific technologies will be illuminated through the collaborative sharing of use-case insights and development of “risk libraries.”
In 2022, addressing organizational challenges to innovation adoption will be front and center, with the recognition that company culture and change-management approaches play vital roles in the ability to adopt new ways of working. Both large and small sponsor organizations will recognize innovation adoption as an industry imperative, as they should. Patients depend on our ability to break down silos, execute clinical trials with a holistic approach, ensure overall quality and leverage new technologies in a way that enhances the clinical trial execution process, making clinical trials accessible to more people and to more diverse patient populations.

Patricia Leuchten
Chief Change Officer
WCG
Clinical Safety and Study Endpoints
Better Harmonization of Safety Data Collection and Reporting
For 2022, I am excited about publication of the Safety Reference Model v1.0 (SRM) by Avoca Quality Consortium, a globally harmonized resource containing interpreted best practices for operationalizing safety reporting in more than 125 countries.
Thirty-three sponsors, CROs and sites have contributed to the reference model and have agreed to standardize upon its implementation and to harmonize approaches. Large CROs have been the biggest backers of this simplification because they deal with so many differences in the way sponsors approach global safety reporting. Gains in quality and efficiency in harmonization more than outweigh the lost revenue from selling regulatory intelligence.
Lack of global harmonization has been the largest problem in safety reporting because there are more than 40 different regulatory frameworks for safety reporting worldwide, which leads to conservative approaches and over-reporting by sponsors who fear regulatory repercussions.
The noteworthy feature of SRM is that it contains executable regulatory intelligence, which can be implemented in processes or integrated into a safety distribution system. This enables precision distribution of safety reports to the right person at the right time anywhere in the world. Janssen, Amgen and Covance have implemented earlier versions of this model and have seen 50 percent reductions in the volume of safety reports distributed.
The topic of over-reporting of safety reports is of special interest to me because I had the privilege of discussing this subject with deputy FDA CDER directors, Robert Temple and Jacqueline Corrigan-Curay, during a recent webinar “A Fresh Perspective from the FDA on the Final IND Safety Reporting Rule.” Most of the major academic cancer centers in the U.S. are currently noncompliant with the FDA IND Safety Reporting final rule because sponsors are still over-reporting SUSARs in oncology by about 85 percent. Recognizing the gravity of the issue, FDA has published two minor updates to guidance this year and are active observers of the SRM initiative.

Steven Beales
Senior Vice President, Scientific and Regulatory Review
WCG
Adjudication of Real-World Data
The events of the past two years have forever changed the pace at which clinical trials are implemented and conducted, with regulators authorizing treatments more quickly than ever in history. This urgency was brought on by the events of the pandemic; however, I suspect it will change the pace of many trials in 2022 and beyond as remote visits and other modifications are adopted.
Despite very well-designed large trials, we need to continue to collect data on recently developed therapeutics after use in the larger public. The evaluation of real-world data will be a significant contributor to the overall knowledge around these authorized products for both safety and efficacy. The use of endpoint adjudication will play an important role in validating this data, especially since data collection is less systematic than that which occurs in a clinical trial. The application of adjudication to this real-world data will add validity to the evidence collected and analyzed. It will be essential to understanding the long-term safety and efficacy of these therapeutics that have been authorized quickly.
In 2022 and beyond, the use of adjudication will facilitate the continued collection of robust data in the era of real-world evidence.

Bindi K. Shah, MD
Chief Medical Officer
WCG ACI Clinical
Advancing the Endpoints to Assess Cognition
Throughout the human developmental lifecycle, cognitive function is one of the most important components of everyday functioning. Impaired cognition is a central feature of many psychiatric and neurological disorders, ranging from schizophrenia to multiple sclerosis to major depression.
The COVID pandemic challenged clinical research that required regular assessment of cognition with paper-and-pencil methods in face-to-face settings. However, this challenge expedited the use of digital, remote assessment technologies and birthed an increased use of inventive strategies that will transform the future of clinical trials.
In 2022, we will see that validation of these measures and an understanding their limitations will remain crucial. Serious researchers must continue to require that any assessment tool demonstrate validity in the clinical populations of interest as regulatory agencies, including the FDA, have made clear that supportive data will be required for methods assessing cognition and related functioning for them to be accepted into mainstream regulatory pathways.
It will also continue to be essential to match any cognitive and functional assessment method to the area of cognition that needs to be assessed and the capacity of the patient population to complete the tests in a valid manner. In 2022, researchers will scale up, using a variety of solutions to support the assessment of cognition and functional capacity and to help determine which tools are scientifically valid and safe to use for clinical trials.

Richard Keefe, PhD
Chief Executive Officer
WCG VeraSci
Scientific Considerations:
Advances in Gene Therapy and Oncology
Genetically-Engineered Immune Effector Cells
Genetically engineered immune effector cells—especially CAR-T cells—will certainly offer many exciting new developments in 2022. The first CAR-T products to receive FDA approval were engineered to attack cellular targets bearing the CD19 antigen on their surfaces. CD19 is a specific marker for B-cells, which are white blood cells that normally play an important role in immunity but can also transform into malignant lymphomas or leukemias requiring complex treatment. Three innovative trends in this space are listed below.
- Aside from cancer pathology, B-cells can also contribute to autoimmune disease by producing pathogenic antibodies. New initiatives are exploring whether CD19-directed CAR-T therapy may be able to treat autoimmune disease by eliminating pathogenic B- cells and the antibodies they produce.
- A broad range of efforts is underway to engineer new classes of cancer-killing viruses, also known as oncolytic viruses. Solid tumors do not express CD19. New research suggests that oncolytic viruses can be engineered to express the CD19 antigen in solid tumors and thus redirect CD19 CAR-T cells to kill the tumor. This approach can potentially leverage FDA-approved CAR-T products to treat a new class of cancers, opening the door to a new category of combination therapy.
- Finally, a major hurdle in delivering CAR-T therapy to patients is the difficulty of ex vivo production. All FDA-approved CAR-T products are produced by extraction of progenitor T-cells from the blood of the cancer patient, followed by genetic engineering of the T-cells in the lab and reinfusion of the engineered cells back into the same patient. It may be possible to use viral or nanoparticle vectors to engineer CAR-T products in vivo. Exciting new efforts are focused on bypassing cellular product manufacturing and directing production of CAR-T therapies in the body of each human patient. If successful, this approach is expected to lead to a dramatic decrease in the time and expense of delivering CAR-T therapies.

Daniel Kavanagh, PhD, RAC
Senior Scientific Advisor, Gene Therapy
WCG IRB
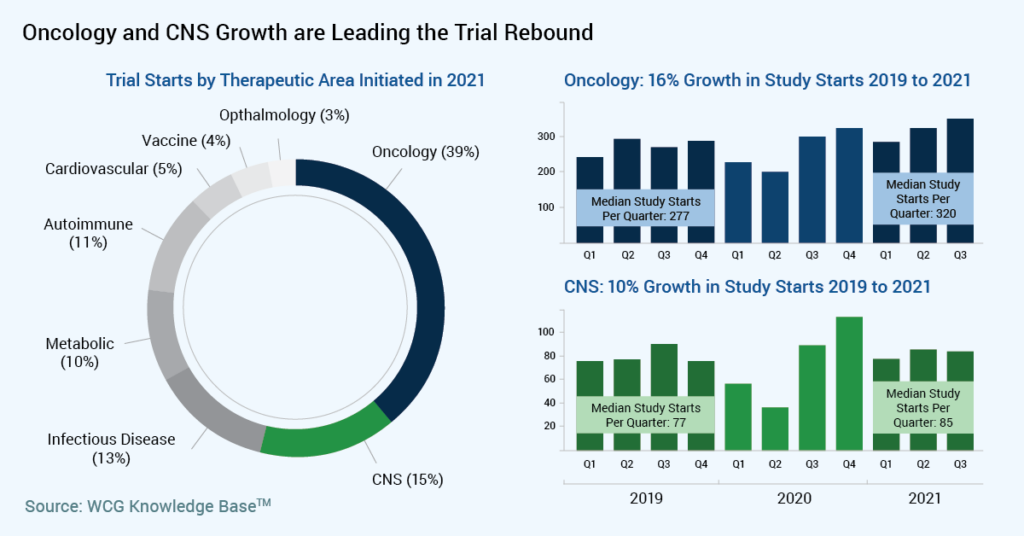
Progress in Oncology Research
Significant progress is being made in oncology care with cancer death rates declining 31% since 1991 and the largest single decline in cancer mortality in the U.S. reported in 2021 from 2017 to 2018 at 2.4%.1 With oncology drugs increasingly receiving expedited reviews, orphan or breakthrough designations, advances will continue at a rapid pace and oncology clinical trials will remain a dominant therapeutic focus.
Experiencing less disruption than other disease states during the pandemic, the initiation of new oncology trials will continue to surge in 2022 with precision therapies at the forefront. Approximately three-fourths of trials have molecular targets,2 further amplifying the use of genomic testing and the identification of more predictive biomarkers to effectively deliver personalized precision treatments.
The ongoing development of small-molecule targeted drugs will remain a major area of cancer research, offering options for more oral therapies. Building on the durable responses of the checkpoint inhibitors, identifying optimal combinations with other complimentary drugs and other treatment modalities, including radiotherapy or surgery, will remain of intense interest together with other mechanisms impacting the immune system.
Finally, biotherapeutic trials will become more accessible including T-cell transfer therapies (CAR-T, TIL, TCR, NK), vaccines, antibody-drug conjugates (ADCs), bispecific antibodies (BiTEs) and premade (off-the shelf) generic antibody products.
Oncology trials are noted for their complexity. The industry has navigated a pandemic while providing continuity of care for participants in clinical trials and contributing to scientific advancements.
In 2022, integrating innovative approaches into clinical trial workflows—including telehealth, artificial intelligence/machine learning, real-world data, hybrid models and other digital tools—will be essential to conducting more trials with fewer patients.
References:
- Cancer Facts and Figures: 2021. Published by the American Cancer Society. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf
- Global Oncology Trends 2021: Outlook to 2025, IQVIA Institute, June 2021. Available at: https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2021

Sandy Smith, RN, MSN, AOCN
Senior Vice President, Clinical Solutions and Strategic Partnerships
WCG
The Patient Voice: Advocacy and Awareness of Under-Represented Populations
Moving Forward in Patient Advocacy
2022 is an unprecedented time for patients at the center of clinical research. The signs are undeniably all around.
- We have had a growing list of rare disease drug approvals for some years, proving the concept that a narrow focus on a few patients can lead to breakthrough therapies;
- Substantive movements toward more equity and inclusivity of clinical trials are well underway and starting to show traction and promise for communities previously isolated from research;
- Common diseases are becoming more subdivided genetically so that trials may be planned more like a rare disease trial with specific DNA variations considered, right down to an N of 1;
- Outcomes when tumor-specific DNA is matched to specific medicine seem more promising than a mass application of one-size-fits-all treatments that sometimes do great harm; and
- Approaches to fixing defective DNA, rather than just addressing symptoms, are on the rise with a growing list of trials using AAV, ASO, CRISPR and other genetic approaches.
We also continue to see the benefits of moving diagnosis closer to the time of birth. A growing list of treatments are being added to the “recommended newborn screening panels,” and exome sequencing is increasingly available in the NICU when something is “not right.” Such screening can reduce or eliminate the diagnostic odyssey that has been so harmful and a drag on a family’s quality of life. This means more of the right treatments, less of the wrong treatments and a reduction in countless visits to clinics in search of a diagnosis.
We will need this decade to work out the science, the medicine, the regulation and the access issues patients face. Questions about where to place our bets and invest our optimism will persist in 2022 and beyond. What is certain is that clinical research is advancing patient centricity, and vice versa.

Steve Smith
President, Patient Advocacy
WCG
Diversity, Equity and Inclusion in Clinical Research
Diversity, Equity and Inclusion (DE&I) in clinical trials has been one of the key themes in 2021 and shows no signs of abating in 2022. For us, the broad appeal of the topic aligns perfectly with our passion for ethics, science and quality. We are embracing this moment in partnership with our biopharmaceutical sponsors and clinical sites to ensure confidence in clinical trial outcomes for all people who might benefit from future treatments.
As scientists, our progress rests on the scientific, academic and professional work of others. We rest on decades of prior efforts by colleagues near and far to help sponsors design DE&I into clinical trial programs.
In 2022, we will see further investment from sponsors on enterprise-level strategies. Critical success factors that will influence DE&I outcomes are identified at the portfolio, disease, asset and trial level.
While barriers exist and are identified, the solutions have become clearer. Here are a few key areas we anticipate seeing in the coming year to improve the accessibility of clinical trials for all:
- Building long-term relationships with trust-bearers in the community;
- Inviting a diverse community of patients and caregivers to the table early and including them throughout the lifecycle of the program or trial;
- Actively listening with humility to the issues and concerns of patients and care providers, remaining as flexible as possible to incorporate their voice into solutions;
- Ensuring diversity strategies are actionable by the study teams and sites;
- Providing sites with the tools necessary to be successful (such as training on implicit bias, cultural competence and health literacy); and
- Increasing site budgets when necessary.
Success will depend on how well sponsors can embed top-down cultural change in clinical science and clinical operations. Some are ahead and some are just beginning, but we have no doubt that sponsors are committed to these changes in 2022 and beyond.

Lori Abrams
Vice President, Patient Advocacy and Clinical Research Diversity
WCG

Emily Ricketts, MSc, PMP
Vice President, Operational Strategy and Planning
WCG
Diversity in Drug Development Programs
The events of the past two years have created an urgent and nonnegotiable imperative to increase diversity in clinical research: diversity in how studies are executed and in the types of patients recruited.
In November 2020, FDA released the guidance document “Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices and Trial Designs Guidance for Industry.” In 2021, sponsors began receiving feedback from FDA on trial submissions asking that they demonstrate how they will ensure appropriate representation of enrolled participants.
As we move forward into 2022 and beyond, the industry must contend with fully building diversity into the clinical operations culture; deciding how much executional diversity to maintain, supporting sites in its maintenance and determining how we can sustainably maintain participant diversity in clinical research. Based on our internal research exploring the relative priority and drivers of patient diversity in clinical research, we see that sponsors who have received specific regulatory feedback need to consider how they will influence trial operations design and new approaches to site operations that will enhance diverse representation among clinical trial participants.

Melissa Bomben
Senior Vice President, Clinical Solutions and Strategic Partnerships
WCG
Request a Meeting with a WCG Expert
Interested to learn how the insights above will affect your studies? Contact us to request a meeting with one of WCG’s consultants.