CROs

Questions on the FDA’s 30-Day IND Review Period and IRB Approval
Blog Posts
Series: Ask the IRB & IBC Experts
Is it possible to make a PI’s name or institution confidential on a consent form?
Blog Posts
Clinical Trial Safety
WCG IBC Services
Solution Overviews
WCG Experts to Lead Nine Educational Sessions at the PRIM&R 2019 Advancing Ethical Research Conference
News
How to Maximize ROI by Implementing a Holistic Recruitment Strategy
Blog Posts
2019 Global Site Relationship Survey – CRO Executive Summary Pages
Solution Overviews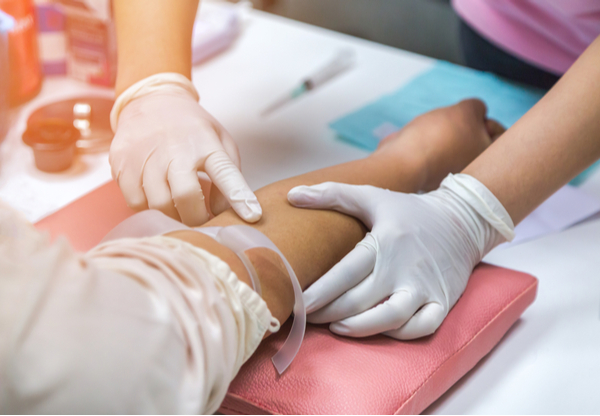
Series: Ask the IRB & IBC Experts
What are the Blood Draw Guidelines for Phase 1 Clinical Trials?
Blog Posts
Questions on Using Generic Recruitment Flyers at Clinical Trial Sites
Blog Posts
Regulatory Compliance
Guidance on genomic research with deceased patients
Blog Posts